CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain - PubMed (original) (raw)
CD8+ T Cells and Endogenous IL-10 Are Required for Resolution of Chemotherapy-Induced Neuropathic Pain
Karen Krukowski et al. J Neurosci. 2016.
Abstract
Chemotherapy-induced peripheral neuropathy (CIPN), characterized by pain and numbness in hands and feet, is a common side effect of cancer treatment. In most patients, symptoms of CIPN subside after treatment completion. However, in a substantial subgroup, CIPN persists long into survivorship. Impairment in pain resolution pathways may explain persistent CIPN. We investigated the contribution of T cells and endogenous interleukin (IL)-10 to resolution of CIPN. Paclitaxel-induced mechanical allodynia was prolonged in T-cell-deficient (Rag1-/-) mice compared with wild-type (WT) mice. There were no differences between WT and Rag1-/- mice in severity of paclitaxel-induced mechanical allodynia. Adoptive transfer of either CD3+ or CD8+, but not CD4+, T cells to Rag1-/- mice normalized resolution of CIPN. Paclitaxel treatment increased the number of T cells in lumbar dorsal root ganglia (DRG), where CD8+ T cells were the major subset. Inhibition of endogenous IL-10 signaling by intrathecal injection of anti-IL-10 to WT mice or Rag1-/- mice reconstituted with CD8+ T cells delayed recovery from paclitaxel-induced mechanical allodynia. Recovery was also delayed in IL-10 knock-out mice. Conversely, administration of exogenous IL-10 attenuated paclitaxel-induced allodynia. In vitro, IL-10 suppressed abnormal paclitaxel-induced spontaneous discharges in DRG neurons. Paclitaxel increased DRG IL-10 receptor expression and this effect requires CD8+ T cells. In conclusion, we identified a novel mechanism for resolution of CIPN that requires CD8+ T cells and endogenous IL-10. We propose that CD8+ T cells increase DRG IL-10 receptor expression and that IL-10 suppresses the abnormal paclitaxel-induced spontaneous discharges by DRG neurons to promote recovery from CIPN.
Significance statement: Chemotherapy-induced peripheral neuropathy persists after completion of cancer treatment in a significant subset of patients, whereas others recover. Persistent neuropathy after completion of cancer treatment severely affects quality of life. We propose that understanding how neuropathy resolves will identify novel avenues for treatment. We identified a novel and critical role for CD8+ T cells and for endogenous IL-10 in recovery from paclitaxel-induced neuropathy in mice. Enhancing the capacity of CD8+ T cells to promote resolution or increasing IL-10 signaling are promising targets for novel interventions. Clinically, peripheral blood CD8+ T-cell function and/or the capacity of individuals to produce IL-10 may represent biomarkers of risk for developing persistent peripheral neuropathy after completion of cancer treatment.
Keywords: CD8+ T cells; IL-10; chemotherapy-induced peripheral neuropathy.
Copyright © 2016 the authors 0270-6474/16/3611074-10$15.00/0.
Figures
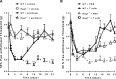
Figure 1.
Contribution of T cells to paclitaxel-induced mechanical allodynia. A, Paclitaxel (2 mg/kg, i.p.) was administered to WT (C57BL/6) and Rag1−/− mice on day 0 and day 2. Mechanical allodynia was measured using von Frey hairs and the 50% paw withdrawal threshold was calculated using the up-and-down method. WT + vehicle (circles, gray line); Rag1−/− + vehicle (open squares, gray dashed line); WT + paclitaxel (diamonds black line); and Rag1−/− + paclitaxel (open triangles, black dashed line). Two-way repeated-measures ANOVA revealed a main effect of time (p < 0.01), a group effect (p < 0.01), a genotype effect (p < 0.01) and a 3-way interaction among group, genotype, and time (p < 0.01). Bonferroni post hoc analysis showed differences between groups (WT + vehicle vs WT + paclitaxel or Rag1−/− + vehicle vs Rag1−/− + paclitaxel) at various time points; n = 8–10/group. **p < 0.01, ***p < 0.001. B, WT and Rag1−/− mice received PBS or CD3+ T cells intravenously 1 d before paclitaxel treatment as in A. Shown are: WT + PBS (circles, gray line); WT + T cells (diamonds, black line); Rag1−/− + PBS (open triangles, gray dashed line); and Rag1−/− + T cells (open squares, black dashed line). Two-way repeated-measures ANOVA revealed a main effect of time (p < 0.01), a group effect (p < 0.01), a treatment effect (T cell vs PBS, p < 0.01), and a 3-way interaction among group, treatment, and time (p < 0.01). Bonferroni post hoc analysis showed differences between groups (WT+ PBS vs WT + PBS or Rag1−/− + PBS vs Rag1−/− + T cells) at various time points; n = 6–8/group. *p < 0.05, **p < 0.01.
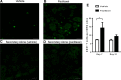
Figure 2.
T-cell localization after paclitaxel treatment. WT mice were treated with paclitaxel or vehicle as in Figure 1_A_ and DRG were collected on day 7. A–D, Representative examples of immunofluorescence analysis of DRG stained with CD3 antibody. Shown are: vehicle treated (A), paclitaxel treated (B), vehicle stained with secondary antibody alone (C), and paclitaxel stained with secondary antibody alone (D). E, Quantification of T-cell numbers/DRG slice. Five slices per mouse were quantified; n = 3–4 mice/group. *p < 0.05, two-way ANOVA followed by Bonferroni post test.. Scale bar, 20 μm.
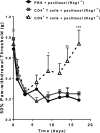
Figure 3.
Identification of the T-cell subset responsible for recovery from paclitaxel-induced mechanical allodynia. Paclitaxel (2 mg/kg, i.p.) was administered to Rag1−/− mice on day 0 and day 2. CD8+ or CD4+ T cells were adoptively transferred to Rag1−/− mice 1 d before the first paclitaxel treatment. Shown are: PBS + paclitaxel (circles, black line); CD4+ T cells + paclitaxel (squares, gray line); and CD8+ T cells (open triangles, dashed black line). Mechanical allodynia was measured as in Figure 1. Two-way repeated-measures ANOVA showed a main effect of time (p < 0.0001), group (p < 0.03), and a group-by-time interaction (p < 0.001). Bonferroni post hoc analysis for CD8+ T cells versus saline: *p < 0.05, **p < 0.01, ***p < 0.001; n = 4–6/group. No significant differences were observed between mice receiving CD4+ T cells or saline.
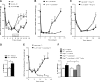
Figure 4.
Impact of endogenous IL-10 on recovery from paclitaxel-induced mechanical allodynia. A, Paclitaxel (2 mg/kg, i.p.) was administered to WT mice on day 0 and day 2. Anti-IL10, anti-TGF-β, or control antibodies (10 μg/mouse/d) were administered intrathecally from days 6–10, and mechanical allodynia was followed over time. Shown are: IgG (circles, gray line); anti-IL-10 (squares, black line); and anti-TGF-β (open triangles, gray dashed line). Two-way repeated-measures ANOVA showed a main effect of time (p < 0. 001), a group effect (p < 0.01), and a group-by-time interaction (p < 0.001). Bonferroni post hoc analysis of anti-IL10 versus isotype-control antibody: ***p < 0.001; n = 8–10/group. B, Paclitaxel (2 mg/kg, i.p.) was administered to IL-10−/− or WT mice on day 0 and day 2. Shown are: IL-10−/− mice (gray circles), WT mice (black squares). Two-way repeated-measures ANOVA showed a main effect of time (p < 0.001), a group effect (p < 0.001), and a group-by-time interaction (p < 0.001). Bonferroni post hoc analysis: ***p < 0.001; n = 6–7/group. C, Paclitaxel (2 mg/kg, i.p.) was administered to Rag1−/− mice on day 0 and day 2. CD8+ cells isolated from WT mice were adoptively transferred to Rag1−/− mice 1 d before the first paclitaxel treatment. Anti-IL10 or control IgG antibodies (10 μg/mouse/d) were administered intrathecally from days 6–10 and mechanical allodynia was followed over time. Shown are: IgG CD8+ Rag1−/− (open circles) and IL-10 CD8+ Rag1−/− (black squares). Two-way repeated-measures ANOVA showed a main effect of time (p < 0.001), a group effect (p < 0.001), and a group-by-time interaction (p < 0.001). Bonferroni post hoc analysis: *p < 0.05, ***p < 0.001; n = 8/group. D, Paclitaxel (2 mg/kg, i.p.) was administered to WT mice on day 0 and day 2. IL-10 mRNA expression levels in lumbar spinal cord were examined on day 7 after paclitaxel treatment. Student's t test measured differences between vehicle-treated (open bars) and paclitaxel-treated (black bars) mice. **p < 0.01; n = 8/group. E, Paclitaxel (2 mg/kg, i.p.) was administered to Rag1−/− mice on day 0 and day 2. CD8+ cells isolated from IL-10−/− mice were adoptively transferred to Rag1−/− mice 1 d before the first paclitaxel treatment and mechanical allodynia was assessed. Shown are: PBS (open circles, gray dashed line) and IL-10−/− + CD8+ T cells (squares, black line). One-way repeated-measures ANOVA showed a main effect of time (p < 0.001), a group effect (p < 0.001), and a group-by-time interaction (p < 0.001). Bonferroni post hoc analysis: **p < 0.01; ***p < 0.001; n = 8/group. F, IL-10 receptor mRNA levels were assessed in lumbar DRG of WT mice, Rag1−/− mice, and Rag1−/− mice reconstituted with CD8+ T cells at day 7 after paclitaxel treatment. Shown are: WT + vehicle (open black bars); WT + paclitaxel (black bars); Rag1−/− + vehicle (open gray bars); Rag1−/− + paclitaxel (gray bars); and Rag1−/− + paclitaxel + CD8+ T cells (patterned bars). One-way ANOVA revealed significant differences between groups. *p < 0.05; n = 6–10/group.
Figure 5.
Effect of IL-10 on paclitaxel-induced mechanical allodynia and spontaneous discharges of DRG neurons. A, IL-10 synerkine (1 μg/mouse) was administered intrathecally from days 5–8 after the start of paclitaxel treatment and mechanical allodynia was monitored. Shown are: IL-10 synerkine (open circles, black dashed line) and saline (squares, gray line). Two-way repeated-measures ANOVA revealed a main effect of time (p < 0.001), a group effect (p < 0.01), and a group-by-time interaction (p < 0.05). Bonferroni post hoc test: **p < 0.01; n = 10/group. B, Lumbar DRG neurons from paclitaxel-treated rats were cultured in vitro. Spontaneous activity was recorded at baseline, after the addition of IL-10, and after washout. C, Bar graphs summarize results obtained in seven cells from four paclitaxel-treated animals.
Comment in
- Roles for CD8+ T Cells and IL-10 in the Resolution of Paclitaxel-Induced Neuropathic Pain.
Bravo-Caparrós I, Nieto FR. Bravo-Caparrós I, et al. J Neurosci. 2017 Mar 15;37(11):2803-2805. doi: 10.1523/JNEUROSCI.3917-16.2017. J Neurosci. 2017. PMID: 28298569 Free PMC article. No abstract available.
Similar articles
- Macrophage Toll-like Receptor 9 Contributes to Chemotherapy-Induced Neuropathic Pain in Male Mice.
Luo X, Huh Y, Bang S, He Q, Zhang L, Matsuda M, Ji RR. Luo X, et al. J Neurosci. 2019 Aug 28;39(35):6848-6864. doi: 10.1523/JNEUROSCI.3257-18.2019. Epub 2019 Jul 3. J Neurosci. 2019. PMID: 31270160 Free PMC article. - DRG Voltage-Gated Sodium Channel 1.7 Is Upregulated in Paclitaxel-Induced Neuropathy in Rats and in Humans with Neuropathic Pain.
Li Y, North RY, Rhines LD, Tatsui CE, Rao G, Edwards DD, Cassidy RM, Harrison DS, Johansson CA, Zhang H, Dougherty PM. Li Y, et al. J Neurosci. 2018 Jan 31;38(5):1124-1136. doi: 10.1523/JNEUROSCI.0899-17.2017. Epub 2017 Dec 18. J Neurosci. 2018. PMID: 29255002 Free PMC article. - Characterisation of Immune and Neuroinflammatory Changes Associated with Chemotherapy-Induced Peripheral Neuropathy.
Makker PG, Duffy SS, Lees JG, Perera CJ, Tonkin RS, Butovsky O, Park SB, Goldstein D, Moalem-Taylor G. Makker PG, et al. PLoS One. 2017 Jan 26;12(1):e0170814. doi: 10.1371/journal.pone.0170814. eCollection 2017. PLoS One. 2017. PMID: 28125674 Free PMC article. - Beyond symptomatic relief for chemotherapy-induced peripheral neuropathy: Targeting the source.
Ma J, Kavelaars A, Dougherty PM, Heijnen CJ. Ma J, et al. Cancer. 2018 Jun 1;124(11):2289-2298. doi: 10.1002/cncr.31248. Epub 2018 Feb 20. Cancer. 2018. PMID: 29461625 Free PMC article. Review. - Mechanisms underlying paclitaxel-induced neuropathic pain: Channels, inflammation and immune regulations.
Xu Y, Jiang Z, Chen X. Xu Y, et al. Eur J Pharmacol. 2022 Oct 15;933:175288. doi: 10.1016/j.ejphar.2022.175288. Epub 2022 Sep 17. Eur J Pharmacol. 2022. PMID: 36122757 Review.
Cited by
- Putative roles of SLC7A5 (LAT1) transporter in pain.
Alles SRA, Gomez K, Moutal A, Khanna R. Alles SRA, et al. Neurobiol Pain. 2020 Jun 30;8:100050. doi: 10.1016/j.ynpai.2020.100050. eCollection 2020 Aug-Dec. Neurobiol Pain. 2020. PMID: 32715162 Free PMC article. Review. - What role of the cGAS-STING pathway plays in chronic pain?
Wu J, Li X, Zhang X, Wang W, You X. Wu J, et al. Front Mol Neurosci. 2022 Aug 1;15:963206. doi: 10.3389/fnmol.2022.963206. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35979145 Free PMC article. Review. - Vincristine-Induced Peripheral Neuropathy in Childhood Acute Lymphoblastic Leukemia: Genetic Variation as a Potential Risk Factor.
Yang QY, Hu YH, Guo HL, Xia Y, Zhang Y, Fang WR, Li YM, Xu J, Chen F, Wang YR, Wang TF. Yang QY, et al. Front Pharmacol. 2021 Dec 9;12:771487. doi: 10.3389/fphar.2021.771487. eCollection 2021. Front Pharmacol. 2021. PMID: 34955843 Free PMC article. Review. - Low-dose interleukin-2 reverses chronic migraine-related sensitizations through peripheral interleukin-10 and transforming growth factor beta-1 signaling.
Guo Z, Zhang J, Liu X, Unsinger J, Hotchkiss RS, Cao YQ. Guo Z, et al. Neurobiol Pain. 2022 Jun 13;12:100096. doi: 10.1016/j.ynpai.2022.100096. eCollection 2022 Aug-Dec. Neurobiol Pain. 2022. PMID: 35733705 Free PMC article. - Neuroimmune Mechanisms Underlying Post-acute Sequelae of SARS-CoV-2 (PASC) Pain, Predictions from a Ligand-Receptor Interactome.
Lesnak JB, Mazhar K, Price TJ. Lesnak JB, et al. Curr Rheumatol Rep. 2023 Sep;25(9):169-181. doi: 10.1007/s11926-023-01107-8. Epub 2023 Jun 10. Curr Rheumatol Rep. 2023. PMID: 37300737 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- R01 NS074999/NS/NINDS NIH HHS/United States
- R01 CA200263/CA/NCI NIH HHS/United States
- R21 CA183736/CA/NCI NIH HHS/United States
- R01 NS073939/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials