Selenoprotein S Reduces Endoplasmic Reticulum Stress-Induced Phosphorylation of Tau: Potential Role in Selenate Mitigation of Tau Pathology - PubMed (original) (raw)
Daniel J Torres 1, Andrea S T Dewing 1, Arlene C Kiyohara 1, Stephanie M Barayuga 1, Miyoko T Bellinger 1, Jane H Uyehara-Lock 2, Lon R White 3, Paula I Moreira 4, Marla J Berry 1, George Perry 5, Frederick P Bellinger 1
Affiliations
- PMID: 27802219
- PMCID: PMC5893862
- DOI: 10.3233/JAD-151208
Selenoprotein S Reduces Endoplasmic Reticulum Stress-Induced Phosphorylation of Tau: Potential Role in Selenate Mitigation of Tau Pathology
Rachel H L H Rueli et al. J Alzheimers Dis. 2017.
Abstract
Previous studies demonstrated that selenium in the form of sodium selenate reduces neurofibrillary tangle formation in Alzheimer's disease models. Hyperphosphorylation of tau, which leads to formation of neurofibrillary tangles in Alzheimer's disease, is increased by endoplasmic reticulum (ER) stress. Selenoprotein S (SelS) is part of an ER membrane complex that removes misfolded proteins from the ER as a means to reduce ER stress. Selenate, as with other forms of selenium, will increase selenoprotein expression. We therefore proposed that increased SelS expression by selenate would contribute to the beneficial actions of selenate in Alzheimer's disease. SelS expression increased with ER stress and decreased under conditions of elevated glucose concentrations in the SH-SY5Y neuronal cell line. Reducing expression of SelS with siRNA promoted cell death in response to ER stress. Selenate increased SelS expression, which significantly correlated with decreased tau phosphorylation. Restricting SelS expression during ER stress conditions increased tau phosphorylation, and also promoted aggregation of phosphorylated tau in neurites and soma. In human postmortem brain, SelS expression coincided with neurofibrillary tangles, but not with amyloid-β plaques. These results indicate that selenate can alter phosphorylation of tau by increasing expression of SelS in Alzheimer's disease and potentially other neurodegenerative disorders.
Keywords: Alzheimer’s disease; endoplasmic reticulum stress; neurofibrillary tangle; selenium; selenoprotein; tau.
Figures
Fig. 1
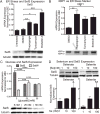
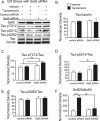
Regulation of expression of SelS in SH-SY5Y cells by ER stress and selenium. A) Above: SelS mRNA measured with qPCR is increased following ER stress induced by tunicamycin, brefeldin A, or thapsigargin. Below: SelS protein (21 kDa) measured by western blot is also increased. Tubulin was used as a loading control. B) Above: XBP1 mRNA expression also increases with ER stress induction. Below: XBP1 splicing occurs following treatment with thapsigargin, brefeldin A, or tunicamycin. C) Glucose increases expression of SelS in SH-SY5Y cells. Above: Quantitative reverse-transcriptase PCR measurements of SelS message levels in cells grown with different glucose levels. Expression of SelS decreases as glucose levels increase. In contrast, expression of another selenoprotein, SelW, is slightly elevated as glucose levels are increased. Below: SelS protein is decreased at higher glucose concentrations, shown by western blot. Alpha-tubulin is shown to demonstrate even loading. D) Sodium selenate (left) and selenite (right) both increase SelS expression. SH-SY5Y cells were grown in 0 Se media for 48 h, followed by supplementation with 1, 10, or 100 nm selenate or selenite for 3 days. Representative blots are shown above. Bar graphs show quantitation of SelS protein levels normalized to tubulin, relative to the 10 nM selenium samples. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
Fig. 2
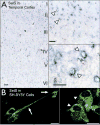
Selenoprotein S in human temporal cortex and SH-SY5Y cells. A) SelS (blue) distribution across cortical layers in the medial temporal cortex (left) and in individual cells. SelS was found in small cells in upper cortical layers as well as in larger pyramidal neurons. Expression was predominantly perinuclear (arrowhead), characteristic of proteins localized to the endoplasmic reticulum. Scale bars: 20 μm. B) SelS in SH-SY5Y neuroblastoma cells, with perinuclear labeling similar to that found in human cortex. SelS was also present in neurites (right, arrow). Scale bars: 20 μm.
Fig. 3
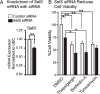
Cell viability of siRNA-transfected neuronal cells measured by MTS assay following challenge with various inducers of ER stress. A) Decrease in SelS mRNA from SelS siRNA. B) Suppression of SelS significantly decreased cell viability of cells for most treatments. ∗p < 0.05, ∗∗p < 0.01, Student’s _t_-test (left) or Tukey’s post hoc test (right), n = 4.
Fig. 4
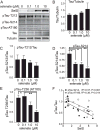
Selenate-induced reductions in tau phosphorylation correlate negatively with SelS expression. SH-SY5Y cells were grown in 0 Se media for 48 h. Following 1 h treatment with 80 nM okadaic acid, cells were grown in 0, 0.1, 1.0, or 10 M sodium selenate for 24 h. A) Western blots for tau phosphorylated at different sites, as well as total tau with tubulin as a loading control. B) Quantitation of tau relative to tubulin, showing no change. C-E) Quantitation of westerns for pTau T212 (C), S214 (D), and S236 (AT180, E). F) Negative correlation of pTau S214 and AT180 relative to SelS. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, Tukey’s post hoc test, n = 4.
Fig. 5
Knockdown of SelS along with ER stress promotes phosphorylation of tau. SelS was knocked down with siRNA in SH-SY5Y cells with and without induction of ER stress by tunicamycin. Phosphorylation of tau was detected with antibodies recognizing tau phosphorylated at specific sites. A) Representative blots with antibodies to SelS, Tau-pT212, pS214, and S262, total tau, and tubulin as a loading control. B) Following knockdown of SelS, the increase in protein expression induced by tunicamycin is significantly reduced. C) Tau-pT212 phosphorylation increases with tunicamycin treatment. D) Tau-pS214 phosphorylation is significantly increased only after SelS knockdown and treatment with tunicamycin. E) There is no significant change in Tau-pS262 phosphorylation. F) Total tau relative to tubulin does not change significantly with ER stress or SelS knockdown. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, Tukey’s post hoc test, n = 4.
Fig. 6
Tunicamycin and SelS siRNA together increase pTau aggregates in cell bodies and neurites. Immunolabeling for Tau-pS214 (green, top row), tau (magenta, second row), or combined (last row) with DAPI (blue). Tunicamycin induced aggregates of pTau in cell bodies (arrowheads), while tunicamycin and SelS siRNA combined also increased pTau aggregates in neuronal processes (arrows). Scale bar: 20 μm.
Fig. 7
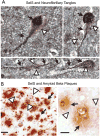
SelS expression is associated with neurofibrillary tangles in human postmortem temporal cortex. A) SelS expression (brown, white arrowheads) is found in cells expressing tangles, as shown by immunoreactivity to tau (black, indicated by black arrows). B) SelS (blue, shown by white arrowheads in top panel) did not appear to have any relationship to amyloid plaques (brown, shown by black arrows). Scale bars: 10 μm (left), 20 μm (right).
Similar articles
- Selenoprotein S is required for clearance of C99 through endoplasmic reticulum-associated degradation.
Jang JK, Park KJ, Lee JH, Ko KY, Kang S, Kim IY. Jang JK, et al. Biochem Biophys Res Commun. 2017 Apr 29;486(2):444-450. doi: 10.1016/j.bbrc.2017.03.060. Epub 2017 Mar 16. Biochem Biophys Res Commun. 2017. PMID: 28315680 - Bip enhanced the association of GSK-3β with tau during ER stress both in vivo and in vitro.
Liu ZC, Fu ZQ, Song J, Zhang JY, Wei YP, Chu J, Han L, Qu N, Wang JZ, Tian Q. Liu ZC, et al. J Alzheimers Dis. 2012;29(4):727-40. doi: 10.3233/JAD-2012-111898. J Alzheimers Dis. 2012. PMID: 22460328 - Selenoprotein S is a marker but not a regulator of endoplasmic reticulum stress in intestinal epithelial cells.
Speckmann B, Gerloff K, Simms L, Oancea I, Shi W, McGuckin MA, Radford-Smith G, Khanna KK. Speckmann B, et al. Free Radic Biol Med. 2014 Feb;67:265-77. doi: 10.1016/j.freeradbiomed.2013.11.001. Epub 2013 Nov 22. Free Radic Biol Med. 2014. PMID: 24275540 - Molecular Mechanisms of ER Stress and UPR in the Pathogenesis of Alzheimer's Disease.
Uddin MS, Tewari D, Sharma G, Kabir MT, Barreto GE, Bin-Jumah MN, Perveen A, Abdel-Daim MM, Ashraf GM. Uddin MS, et al. Mol Neurobiol. 2020 Jul;57(7):2902-2919. doi: 10.1007/s12035-020-01929-y. Epub 2020 May 19. Mol Neurobiol. 2020. PMID: 32430843 Review. - Senescence may mediate conversion of tau phosphorylation-induced apoptotic escape to neurodegeneration.
Wang JZ, Wang ZH. Wang JZ, et al. Exp Gerontol. 2015 Aug;68:82-6. doi: 10.1016/j.exger.2015.03.007. Epub 2015 Mar 14. Exp Gerontol. 2015. PMID: 25777063 Review.
Cited by
- Roles of Selenoproteins in Brain Function and the Potential Mechanism of Selenium in Alzheimer's Disease.
Zhang ZH, Song GL. Zhang ZH, et al. Front Neurosci. 2021 Mar 8;15:646518. doi: 10.3389/fnins.2021.646518. eCollection 2021. Front Neurosci. 2021. PMID: 33762907 Free PMC article. Review. - Effect of single nucleotide polymorphisms in SEPS1 and SEPP1 on expression in the protein level in metabolic syndrome in subjects with cardiovascular disease.
Gharipour M, Ouguerram K, Nazih EH, Salehi M, Behmanesh M, Razavi R, Gharipour A, Diantkhah M, Sadeghi M. Gharipour M, et al. Mol Biol Rep. 2019 Dec;46(6):5685-5693. doi: 10.1007/s11033-019-05000-5. Epub 2019 Sep 21. Mol Biol Rep. 2019. PMID: 31542866 - A selenium species in cerebrospinal fluid predicts conversion to Alzheimer's dementia in persons with mild cognitive impairment.
Vinceti M, Chiari A, Eichmüller M, Rothman KJ, Filippini T, Malagoli C, Weuve J, Tondelli M, Zamboni G, Nichelli PF, Michalke B. Vinceti M, et al. Alzheimers Res Ther. 2017 Dec 19;9(1):100. doi: 10.1186/s13195-017-0323-1. Alzheimers Res Ther. 2017. PMID: 29258624 Free PMC article. - A multi-ethnic proteomic profiling analysis in Alzheimer's disease identifies the disparities in dysregulation of proteins and pathogenesis.
Tan MS, Cheah PL, Chin AV, Looi LM, Chang SW. Tan MS, et al. PeerJ. 2024 Jul 18;12:e17643. doi: 10.7717/peerj.17643. eCollection 2024. PeerJ. 2024. PMID: 39035156 Free PMC article. - "Alphabet" Selenoproteins: Their Characteristics and Physiological Roles.
Dogaru CB, Muscurel C, Duță C, Stoian I. Dogaru CB, et al. Int J Mol Sci. 2023 Nov 6;24(21):15992. doi: 10.3390/ijms242115992. Int J Mol Sci. 2023. PMID: 37958974 Free PMC article. Review.
References
- Corcoran NM, Martin D, Hutter-Paier B, Windisch M, Nguyen T, Nheu L, Sundstrom LE, Costello AJ, Hovens CM. Sodium selenate specifically activates PP2A phosphatase, dephosphorylates tau and reverses memory deficits in an Alzheimer’s disease model. J Clin Neurosci. 2010;17:1025–1033. - PubMed
- Bosse AC, Pallauf J, Hommel B, Sturm M, Fischer S, Wolf NM, Mueller AS. Impact of selenite and selenate on differentially expressed genes in rat liver examined by microarray analysis. Biosci Rep. 2011;30:293–306. - PubMed
- Hill KE, Xia Y, Akesson B, Boeglin ME, Burk RF. Selenoprotein P concentration in plasma is an index of selenium status in selenium-deficient and selenium-supplemented Chinese subjects. J Nutr. 1996;126:138–145. - PubMed
- Persson-Moschos M, Alfthan G, Akesson B. Plasma selenoprotein P levels of healthy males in different selenium status after oral supplementation with different forms of selenium. Eur J Clin Nutr. 1998;52:363–367. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources