A Two-Component Regulatory System Impacts Extracellular Membrane-Derived Vesicle Production in Group A Streptococcus - PubMed (original) (raw)
A Two-Component Regulatory System Impacts Extracellular Membrane-Derived Vesicle Production in Group A Streptococcus
Ulrike Resch et al. mBio. 2016.
Abstract
Export of macromolecules via extracellular membrane-derived vesicles (MVs) plays an important role in the biology of Gram-negative bacteria. Gram-positive bacteria have also recently been reported to produce MVs; however, the composition and mechanisms governing vesiculogenesis in Gram-positive bacteria remain undefined. Here, we describe MV production in the Gram-positive human pathogen group A streptococcus (GAS), the etiological agent of necrotizing fasciitis and streptococcal toxic shock syndrome. M1 serotype GAS isolates in culture exhibit MV structures both on the cell wall surface and in the near vicinity of bacterial cells. A comprehensive analysis of MV proteins identified both virulence-associated protein substrates of the general secretory pathway in addition to "anchorless surface proteins." Characteristic differences in the contents, distributions, and fatty acid compositions of specific lipids between MVs and GAS cell membrane were also observed. Furthermore, deep RNA sequencing of vesicular RNAs revealed that GAS MVs contained differentially abundant RNA species relative to bacterial cellular RNA. MV production by GAS strains varied in a manner dependent on an intact two-component system, CovRS, with MV production negatively regulated by the system. Modulation of MV production through CovRS was found to be independent of both GAS cysteine protease SpeB and capsule biosynthesis. Our data provide an explanation for GAS secretion of macromolecules, including RNAs, lipids, and proteins, and illustrate a regulatory mechanism coordinating this secretory response.
Importance: Group A streptococcus (GAS) is a Gram-positive bacterial pathogen responsible for more than 500,000 deaths annually. Establishment of GAS infection is dependent on a suite of proteins exported via the general secretory pathway. Here, we show that GAS naturally produces extracellular vesicles with a unique lipid composition that are laden with proteins and RNAs. Interestingly, both virulence-associated proteins and RNA species were found to be differentially abundant in vesicles relative to the bacteria. Furthermore, we show that genetic disruption of the virulence-associated two-component regulator CovRS leads to an increase in vesicle production. This study comprehensively describes the protein, RNA, and lipid composition of GAS-secreted MVs and alludes to a regulatory system impacting this process.
Copyright © 2016 Resch et al.
Figures
FIG 1
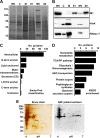
GAS cells exhibit MV protrusions during in vitro growth. Cellular morphologies of ISS3348 (A, C, and E) and SF370 (B, D, and F) were examined by SEM (A and B), AFM (C and D), and TEM (E and F). Isolated MVs from ISS3348 were visualized using negative-stain TEM (G) and staining for M1 protein and STED microscopy (H). White arrowheads indicate vesicle-like structures. Scale bars are drawn to 200 nm (A and B and E to G) or 500 nm (C, D, and H).
FIG 2
GAS MVs carry classically secreted and anchorless virulence proteins. (A) Protein profiles of ISS3348 whole-cell extract (WC), cytoplasm (C), membrane (M), secreted fraction prior to ultracentrifugation (S1), MV, and secreted fraction after centrifugation (S2). (B) Western blot of virulence-associated proteins identified in MVs. The samples in each lane are the same as in panel A. (C) Swiss-Prot classification of nano-LC-MS/MS-identified MV proteins. (D) KEGG categories enriched in MV proteins. (E) Silver-stained 2D protein profile and respective 2D Western blot of ISS3348 MV proteins probed with ARF patient antisera and anti-human IgG-Fc-horseradish peroxidase (HRP).
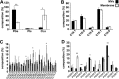
FIG 3
PG is the dominant anionic phospholipid in GAS MVs. (A) Characterization of anionic phospholipids phosphatidylglycerol (PG), phosphatidylinositol (PI), and cardiolipin (CL) in ISS3348 MVs and membranes. (B) General distribution of GAS medium-chain fatty acids (FAs) in MVs and membranes. (C and D) Comparison of GAS MVs and membrane FA saturation level and arrangement in anionic lipids CL and PG. The results presented are means ± standard deviations (SD) from 3 experiments. Asterisks indicate statistical significance by one-way analysis of variance (ANOVA) with Tukey’s post hoc test: *, P < 0.05; **, P < 0.01.
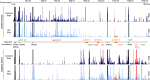
FIG 4
RNA species are differentially abundant between GAS cells and MVs. Coverage of reads from MVs and bacterial cellular RNAs mapped to the reference M5005 genome. Chromosomal coordinates are indicated at the top in kilobases. The scale for mapped reads is indicated to the right of the coverage map. tRNA and rRNA loci are indicated in black. The positions of prophage regions are indicated in orange. Selected RNA species more abundant in MVs are highlighted in green, and RNA species less abundant in MVs are highlighted in red. The vesicular and bacterial coverages shown are from a representative sample from biological triplicates.
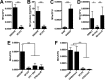
FIG 5
CovRS signaling impacts MV production. Shown is quantification of MV production by GAS strains on the basis of FM1-43 dye staining expressed as MVs per CFU (A and C to F) or total vesicular protein abundance estimated by Bradford protein determination expressed as femtograms of MV protein per CFU (B). Asterisks indicate statistical significance by Student’s unpaired t test (A to C) or one-way ANOVA with Tukey’s post hoc test (D to F): *, P < 0.05; **, P < 0.01; and, ***, P < 0.001. The results presented are means ± SD from 3 to 5 independent experiments, each of which was conducted with 2 technical replicates.
Similar articles
- CovRS-Regulated Transcriptome Analysis of a Hypervirulent M23 Strain of Group A Streptococcus pyogenes Provides New Insights into Virulence Determinants.
Bao YJ, Liang Z, Mayfield JA, Lee SW, Ploplis VA, Castellino FJ. Bao YJ, et al. J Bacteriol. 2015 Oct;197(19):3191-205. doi: 10.1128/JB.00511-15. Epub 2015 Jul 27. J Bacteriol. 2015. PMID: 26216843 Free PMC article. - A natural inactivating mutation in the CovS component of the CovRS regulatory operon in a pattern D Streptococcal pyogenes strain influences virulence-associated genes.
Liang Z, Zhang Y, Agrahari G, Chandrahas V, Glinton K, Donahue DL, Balsara RD, Ploplis VA, Castellino FJ. Liang Z, et al. J Biol Chem. 2013 Mar 1;288(9):6561-73. doi: 10.1074/jbc.M112.442657. Epub 2013 Jan 13. J Biol Chem. 2013. PMID: 23316057 Free PMC article. - Complement-mediated opsonization of invasive group A Streptococcus pyogenes strain AP53 is regulated by the bacterial two-component cluster of virulence responder/sensor (CovRS) system.
Agrahari G, Liang Z, Mayfield JA, Balsara RD, Ploplis VA, Castellino FJ. Agrahari G, et al. J Biol Chem. 2013 Sep 20;288(38):27494-27504. doi: 10.1074/jbc.M113.494864. Epub 2013 Aug 8. J Biol Chem. 2013. PMID: 23928307 Free PMC article. Clinical Trial. - From transcription to activation: how group A streptococcus, the flesh-eating pathogen, regulates SpeB cysteine protease production.
Carroll RK, Musser JM. Carroll RK, et al. Mol Microbiol. 2011 Aug;81(3):588-601. doi: 10.1111/j.1365-2958.2011.07709.x. Epub 2011 Jun 24. Mol Microbiol. 2011. PMID: 21707787 Review. - The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci.
Churchward G. Churchward G. Mol Microbiol. 2007 Apr;64(1):34-41. doi: 10.1111/j.1365-2958.2007.05649.x. Mol Microbiol. 2007. PMID: 17376070 Review.
Cited by
- Staphylococcus aureus secretes immunomodulatory RNA and DNA via membrane vesicles.
Rodriguez BV, Kuehn MJ. Rodriguez BV, et al. Sci Rep. 2020 Oct 26;10(1):18293. doi: 10.1038/s41598-020-75108-3. Sci Rep. 2020. PMID: 33106559 Free PMC article. - The extracellular vesicle generation paradox: a bacterial point of view.
McMillan HM, Kuehn MJ. McMillan HM, et al. EMBO J. 2021 Nov 2;40(21):e108174. doi: 10.15252/embj.2021108174. Epub 2021 Oct 11. EMBO J. 2021. PMID: 34636061 Free PMC article. Review. - Pneumococcal Extracellular Vesicles Modulate Host Immunity.
Yerneni SS, Werner S, Azambuja JH, Ludwig N, Eutsey R, Aggarwal SD, Lucas PC, Bailey N, Whiteside TL, Campbell PG, Hiller NL. Yerneni SS, et al. mBio. 2021 Aug 31;12(4):e0165721. doi: 10.1128/mBio.01657-21. Epub 2021 Jul 13. mBio. 2021. PMID: 34253061 Free PMC article. - Lactic acid bacteria derived extracellular vesicles: emerging bioactive nanoparticles in modulating host health.
Li M, Mao B, Tang X, Zhang Q, Zhao J, Chen W, Cui S. Li M, et al. Gut Microbes. 2024 Jan-Dec;16(1):2427311. doi: 10.1080/19490976.2024.2427311. Epub 2024 Nov 13. Gut Microbes. 2024. PMID: 39538968 Free PMC article. Review. - Extracellular Vesicle Biogenesis and Functions in Gram-Positive Bacteria.
Briaud P, Carroll RK. Briaud P, et al. Infect Immun. 2020 Nov 16;88(12):e00433-20. doi: 10.1128/IAI.00433-20. Print 2020 Nov 16. Infect Immun. 2020. PMID: 32989035 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
Funding was also provided to Emmanuelle Charpentier by the Max Planck Society and by the Louis Jeantet Prize for Medicine given by the Louis-Jeantet Foundation and the Göran Gustafsson Prize given by the Göran Gustafsson Foundation.
LinkOut - more resources
Full Text Sources
Other Literature Sources