Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location - PubMed (original) (raw)
doi: 10.1038/ctg.2016.53.
Yin Cao 2 3 4, Andrew T Chan 2 3 5, Zhi Rong Qian 1, Jonathan A Nowak 6, Yohei Masugi 1, Yan Shi 1, Mingyang Song 2 3 4, Annacarolina da Silva 1, Mancang Gu 1, Wanwan Li 1, Tsuyoshi Hamada 1, Keisuke Kosumi 1, Akiko Hanyuda 1, Li Liu 1, Aleksandar D Kostic 7 8 9, Marios Giannakis 1 8 10, Susan Bullman 1 8, Caitlin A Brennan 11, Danny A Milner 6 11, Hideo Baba 12, Levi A Garraway 1 8 10, Jeffrey A Meyerhardt 1, Wendy S Garrett 1 8 11, Curtis Huttenhower 7 8 13, Matthew Meyerson 1 8, Edward L Giovannucci 4 5 14, Charles S Fuchs 1 5, Reiko Nishihara 1 4 7 14, Shuji Ogino 1 6 14
Affiliations
- PMID: 27811909
- PMCID: PMC5543402
- DOI: 10.1038/ctg.2016.53
Fusobacterium nucleatum in Colorectal Carcinoma Tissue According to Tumor Location
Kosuke Mima et al. Clin Transl Gastroenterol. 2016.
Abstract
Objectives: Evidence suggests a possible role of Fusobacterium nucleatum in colorectal carcinogenesis, especially in right-sided proximal colorectum. Considering a change in bowel contents and microbiome from proximal to distal colorectal segments, we hypothesized that the proportion of colorectal carcinoma enriched with F. nucleatum might gradually increase along the bowel subsites from rectum to cecum.
Methods: A retrospective, cross-sectional analysis was conducted on 1,102 colon and rectal carcinomas in molecular pathological epidemiology databases of the Nurses' Health Study and the Health Professionals Follow-up Study. We measured the amount of F. nucleatum DNA in colorectal tumor tissue using a quantitative PCR assay and equally dichotomized F. nucleatum-positive cases (high vs. low). We used multivariable logistic regression analysis to examine the relationship of a bowel subsite variable (rectum, rectosigmoid junction, sigmoid colon, descending colon, splenic flexure, transverse colon, hepatic flexure, ascending colon, and cecum) with the amount of F. nucleatum.
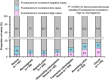
Results: The proportion of F. nucleatum-high colorectal cancers gradually increased from rectal cancers (2.5%; 4/157) to cecal cancers (11%; 19/178), with a statistically significant linear trend along all subsites (P<0.0001) and little evidence of non-linearity. The proportion of F. nucleatum-low cancers was higher in rectal, ascending colon, and cecal cancers than in cancers of middle segments.
Conclusions: The proportion of F. nucleatum-high colorectal cancers gradually increases from rectum to cecum. Our data support the colorectal continuum model that reflects pathogenic influences of the gut microbiota on neoplastic and immune cells and challenges the prevailing two-colon (proximal vs. distal) dichotomy paradigm.
Conflict of interest statement
Guarantor of the article: Shuji Ogino, MD, PhD, MS.
Specific author contributions: All authors contributed to review and revision. Kosuke Mima, Caitlin A. Brennan, Danny A. Milner, Levi A. Garraway, Jeffrey A. Meyerhardt, Wendy S. Garrett, Curtis Huttenhower, Matthew Meyerson, Edward L. Giovannucci, Andrew T. Chan, Charles S. Fuchs, and Shuji Ogino developed the main concept and designed the study. Andrew T. Chan, Charles S. Fuchs, and Shuji Ogino wrote grant applications. Kosuke Mima, Yin Cao, Reiko Nishihara, Zhi Rong Qian, Jonathan A. Nowak, Yohei Masugi, Yan Shi, Annacarolina da Silva, Mancang Gu, Wanwan Li, Tsuyoshi Hamada, Keisuke Kosumi, Akiko Hanyuda, Li Liu, Mingyang Song, Jeffrey A. Meyerhardt, Edward L. Giovannucci, Andrew T. Chan, Charles S. Fuchs, and Shuji Ogino were responsible for collection of tumor tissue and acquisition of epidemiological, clinical and tumor tissue data, including histopathological and immunohistochemical characteristics. Kosuke Mima, Aleksandar D. Kostic, Susan Bullman, Caitlin A. Brennan, Wendy S. Garrett, Curtis Huttenhower, Matthew Meyerson, Charles S. Fuchs, and Shuji Ogino performed data analysis and interpretation. Kosuke Mima, Yin Cao, Reiko Nishihara, and Shuji Ogino drafted the manuscript. Yin Cao, Andrew T. Chan, Mingyang Song, Marios Giannakis, Caitlin A. Brennan, Hideo Baba, Wendy S. Garrett, Matthew Meyerson, Jeffrey A. Meyerhardt, Edward L. Giovannucci, Charles S. Fuchs, Reiko Nishihara, and Shuji Ogino contributed to editing and critical revision for important intellectual contents. All authors approved the final draft submitted.
Financial support: This work was supported by US National Institutes of Health (NIH) grants (P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; P50 CA127003 to C.S.F.; R01 CA137178 to A.T.C.; R01 CA151993 to S.O.; R35 CA197735 to S.O.; and K07 CA190673 to R.N.); and by grants from the Project P Fund, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. K.M. is supported by a grant from Program for Advancing Strategic International Networks to Accelerate the Circulation of Talented Researchers from Japan Society for the Promotion of Science. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Potential competing interests: Chan previously served as a consultant for Bayer Healthcare, Millennium Pharmaceuticals, Pozen, and Pfizer. This study was not funded by Bayer Healthcare, Millennium Pharmaceuticals, Pozen, or Pfizer. This is redundant, and we will keep the last sentence. Meyerson has applied for a patent on Fusobacterium in colorectal cancer diagnosis and had ownership interest in and was a consultant and advisory board member for Foundation Medicine. He also receives research support from Bayer. The other authors declare no competing financial interest.
Figures
Figure 1
Proportions of _Fusobacterium nucleatum_-negative, _F. nucleatum_-low, and _F. nucleatum_-high colorectal carcinoma cases along the bowel subsites. _P_-value was calculated by the linear trend test across the bowel subsite variable (population average distance from anal verge to each subsite (cm)) as a continuous variable in the univariable logistic regression model to predict the amount of tissue F. nucleatum (as a binary outcome variable (high vs. low/negative)).
Similar articles
- Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum.
Yamauchi M, Morikawa T, Kuchiba A, Imamura Y, Qian ZR, Nishihara R, Liao X, Waldron L, Hoshida Y, Huttenhower C, Chan AT, Giovannucci E, Fuchs C, Ogino S. Yamauchi M, et al. Gut. 2012 Jun;61(6):847-54. doi: 10.1136/gutjnl-2011-300865. Epub 2012 Mar 17. Gut. 2012. PMID: 22427238 Free PMC article. - Fusobacterium nucleatum and T Cells in Colorectal Carcinoma.
Mima K, Sukawa Y, Nishihara R, Qian ZR, Yamauchi M, Inamura K, Kim SA, Masuda A, Nowak JA, Nosho K, Kostic AD, Giannakis M, Watanabe H, Bullman S, Milner DA, Harris CC, Giovannucci E, Garraway LA, Freeman GJ, Dranoff G, Chan AT, Garrett WS, Huttenhower C, Fuchs CS, Ogino S. Mima K, et al. JAMA Oncol. 2015 Aug;1(5):653-61. doi: 10.1001/jamaoncol.2015.1377. JAMA Oncol. 2015. PMID: 26181352 Free PMC article. - Association of Fusobacterium nucleatum with clinical and molecular features in colorectal serrated pathway.
Ito M, Kanno S, Nosho K, Sukawa Y, Mitsuhashi K, Kurihara H, Igarashi H, Takahashi T, Tachibana M, Takahashi H, Yoshii S, Takenouchi T, Hasegawa T, Okita K, Hirata K, Maruyama R, Suzuki H, Imai K, Yamamoto H, Shinomura Y. Ito M, et al. Int J Cancer. 2015 Sep 15;137(6):1258-68. doi: 10.1002/ijc.29488. Epub 2015 Mar 13. Int J Cancer. 2015. PMID: 25703934 - Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer.
Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H, Kanno S, Yamamoto I, Ishigami K, Igarashi H, Maruyama R, Imai K, Yamamoto H, Shinomura Y. Nosho K, et al. World J Gastroenterol. 2016 Jan 14;22(2):557-66. doi: 10.3748/wjg.v22.i2.557. World J Gastroenterol. 2016. PMID: 26811607 Free PMC article. Review. - Fusobacterium nucleatum and colorectal cancer: A review.
Shang FM, Liu HL. Shang FM, et al. World J Gastrointest Oncol. 2018 Mar 15;10(3):71-81. doi: 10.4251/wjgo.v10.i3.71. World J Gastrointest Oncol. 2018. PMID: 29564037 Free PMC article. Review.
Cited by
- The relationship between gastrointestinal cancers and the microbiota.
LaCourse KD, Johnston CD, Bullman S. LaCourse KD, et al. Lancet Gastroenterol Hepatol. 2021 Jun;6(6):498-509. doi: 10.1016/S2468-1253(20)30362-9. Epub 2021 Mar 18. Lancet Gastroenterol Hepatol. 2021. PMID: 33743198 Free PMC article. Review. - Differences Regarding the Molecular Features and Gut Microbiota Between Right and Left Colon Cancer.
Kim K, Castro EJT, Shim H, Advincula JVG, Kim YW. Kim K, et al. Ann Coloproctol. 2018 Dec;34(6):280-285. doi: 10.3393/ac.2018.12.17. Epub 2018 Dec 31. Ann Coloproctol. 2018. PMID: 30630301 Free PMC article. - Colorectal Cancer Biomarkers in the Era of Personalized Medicine.
Patel JN, Fong MK, Jagosky M. Patel JN, et al. J Pers Med. 2019 Jan 14;9(1):3. doi: 10.3390/jpm9010003. J Pers Med. 2019. PMID: 30646508 Free PMC article. Review. - Prognostic Impact of Fusobacterium nucleatum Depends on Combined Tumor Location and Microsatellite Instability Status in Stage II/III Colorectal Cancers Treated with Adjuvant Chemotherapy.
Oh HJ, Kim JH, Bae JM, Kim HJ, Cho NY, Kang GH. Oh HJ, et al. J Pathol Transl Med. 2019 Jan;53(1):40-49. doi: 10.4132/jptm.2018.11.29. Epub 2018 Dec 26. J Pathol Transl Med. 2019. PMID: 30586952 Free PMC article. - Detection of Fusobacterium nucleatum DNA in primary care patient stool samples does not predict progression of colorectal neoplasia.
Aitchison A, Pearson JF, Purcell RV, Frizelle FA, Keenan JI. Aitchison A, et al. PLoS One. 2022 Jun 3;17(6):e0269541. doi: 10.1371/journal.pone.0269541. eCollection 2022. PLoS One. 2022. PMID: 35658028 Free PMC article.
References
- Louis P, Hold GL, Flint HJ. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol 2014; 12: 661–672. - PubMed
- Belcheva A, Irrazabal T, Robertson SJ et al. Gut microbial metabolism drives transformation of MSH2-deficient colon epithelial cells. Cell 2014; 158: 288–299. - PubMed
Grants and funding
- KL2 TR001100/TR/NCATS NIH HHS/United States
- R35 CA197735/CA/NCI NIH HHS/United States
- R01 CA118553/CA/NCI NIH HHS/United States
- P01 CA087969/CA/NCI NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- P01 CA055075/CA/NCI NIH HHS/United States
- R01 CA151993/CA/NCI NIH HHS/United States
- R01 CA137178/CA/NCI NIH HHS/United States
- R01 CA154426/CA/NCI NIH HHS/United States
- UM1 CA186107/CA/NCI NIH HHS/United States
- R01 CA169141/CA/NCI NIH HHS/United States
- UM1 CA167552/CA/NCI NIH HHS/United States
- P50 CA127003/CA/NCI NIH HHS/United States
- K07 CA190673/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources