Stem Cell Extracellular Vesicles: Extended Messages of Regeneration - PubMed (original) (raw)
Review
Stem Cell Extracellular Vesicles: Extended Messages of Regeneration
Milad Riazifar et al. Annu Rev Pharmacol Toxicol. 2017.
Abstract
Stem cells are critical to maintaining steady-state organ homeostasis and regenerating injured tissues. Recent intriguing reports implicate extracellular vesicles (EVs) as carriers for the distribution of morphogens and growth and differentiation factors from tissue parenchymal cells to stem cells, and conversely, stem cell-derived EVs carrying certain proteins and nucleic acids can support healing of injured tissues. We describe approaches to make use of engineered EVs as technology platforms in therapeutics and diagnostics in the context of stem cells. For some regenerative therapies, natural and engineered EVs from stem cells may be superior to single-molecule drugs, biologics, whole cells, and synthetic liposome or nanoparticle formulations because of the ease of bioengineering with multiple factors while retaining superior biocompatibility and biostability and posing fewer risks for abnormal differentiation or neoplastic transformation. Finally, we provide an overview of current challenges and future directions of EVs as potential therapeutic alternatives to cells for clinical applications.
Keywords: bioengineering; drug delivery; exosomes; extracellular vesicles; microvesicles; stem cells.
Figures
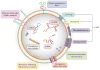
Figure 1
Biogenesis of extracellular vesicles (EVs). Microvesicles derive from the plasma membrane in a way reminiscent of the reverse of endocytosis. Exosomes are generated as intraluminal vesicles (ILVs) inside multivesicular bodies (MVBs), which, in turn, originate either from invaginations of the plasma membrane or from intracellular organelle membranes. Exosomes are created during two successive membrane invaginations: Membranes invaginate inward to generate MVBs, which in turn invaginate inward to generate ILVs.
Figure 2
Anatomy of EVs. EVs contain several characteristic lipids, proteins, and RNA molecules depicted here schematically. Because exosomes are generated when ILVs are released upon MVB fusion with the plasma membrane, exosomes have the same membrane leaflet composition as ILVs. Surface proteins include MHC molecules, ICAMs, integrins, tetraspanins (e.g., CD63, CD81), TSG101, and ALIX. Lumen proteins include cytoskeletal actin and tubulin, Rab GTPases, and inner membrane leaflet-associated proteins. RNAs include mRNA, lncRNA, miRNA, piRNA, vaultRNA, Y-RNA, rRNA, and tRNA (not all depicted here). During MVB biogenesis and development, ILVs may incorporate additional lipids, proteins, and nucleic acids in a nonspecific bystander way or by specific recruitment. Abbreviations: EV, extracellular vesicle; ICAM, intercellular adhesion molecule; ILV, intraluminal vesicle; lncRNA, long noncoding RNA; MHCI, major histocompatibility complex class I; miRNA, microRNA; mRNA, messenger RNA; MVB, multivesicular body; piRNA, picoRNA; rRNA, ribosomal RNA; tRNA, transfer RNA.
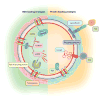
Figure 3
EVs in bidirectional communication between stem and parenchymal cells. Stem cells may sense parenchymal cell injury or distress by receiving parenchymal EVs, and in turn, stem cell EVs harboring prohealing RNAs and proteins may be received by parenchymal cells, maintaining tissue homeostasis. Cellular cytonemes and cilia can establish direct contact between cells for transfer of biomolecules and can also serve as transmission points for EVs. During development, cytonemes and EVs also carry morphogens such as Wnt, Hh, and Notch ligands. Abbreviations: EV, extracellular vesicle; ICAM, intercellular adhesion molecule; MHCI, major histocompatibility complex class I.
Figure 4
Bioengineering EVs for targeted therapy. EVs can be engineered to incorporate nucleic acids and POIs. To concentrate a protein in the lumen of EVs, its interacting partner can be fused with tetraspanin CD63. Likewise, miRNAs can be enriched into EVs by fusing Ago protein with CD63. Poly A binding protein, which binds mature mRNAs, can recruit mRNAs selectively into EVs. lncRNAs can be enriched in EVs by fusing motifs from polycomb repressive complex 2 (EZH1 and EZH2) with tetraspanins. To incorporate POIs on the membranes of EVs, sequences coding for acylation motifs or membrane-spanning helices can be fused to the POIs. Abbreviations: EV, extracellular vesicle; lncRNA, long noncoding RNA; miRNA, microRNA; POI, protein of interest.
Figure 5
Stem cell extracellular vesicles (EVs) for clinical applications. EVs are designed, manufactured, and quality controlled beforehand and stored as off-the-shelf medications to be infused in patients as needed. Other EVs may also be customized accordingly for the individual patient by harvesting autologous stem cells, expanding and modifying them, producing EVs, and infusing them back to the same patient. In this process, the stem cells can also be genetically modified, and their EVs may undergo further modification by loading them with therapeutic molecules. Finally, EVs need to undergo quality control and be stored for future administration.
Similar articles
- Stem cell-derived extracellular vesicles: role in oncogenic processes, bioengineering potential, and technical challenges.
Ullah M, Qiao Y, Concepcion W, Thakor AS. Ullah M, et al. Stem Cell Res Ther. 2019 Nov 26;10(1):347. doi: 10.1186/s13287-019-1468-6. Stem Cell Res Ther. 2019. PMID: 31771657 Free PMC article. Review. - Focus on Extracellular Vesicles: Therapeutic Potential of Stem Cell-Derived Extracellular Vesicles.
Zhang B, Yeo RW, Tan KH, Lim SK. Zhang B, et al. Int J Mol Sci. 2016 Feb 6;17(2):174. doi: 10.3390/ijms17020174. Int J Mol Sci. 2016. PMID: 26861305 Free PMC article. Review. - Human Pluripotent Stem Cell-Derived Extracellular Vesicles: Characteristics and Applications.
Jeske R, Bejoy J, Marzano M, Li Y. Jeske R, et al. Tissue Eng Part B Rev. 2020 Apr;26(2):129-144. doi: 10.1089/ten.TEB.2019.0252. Epub 2020 Jan 16. Tissue Eng Part B Rev. 2020. PMID: 31847715 Free PMC article. Review. - Transfer of Mammary Gland-forming Ability Between Mammary Basal Epithelial Cells and Mammary Luminal Cells via Extracellular Vesicles/Exosomes.
Lin MC, Chen SY, He PL, Luo WT, Li HJ. Lin MC, et al. J Vis Exp. 2017 Jun 3;(124):55736. doi: 10.3791/55736. J Vis Exp. 2017. PMID: 28605392 Free PMC article. - Stem Cells-Derived Extracellular Vesicles: Potential Therapeutics for Wound Healing in Chronic Inflammatory Skin Diseases.
Manchon E, Hirt N, Bouaziz JD, Jabrane-Ferrat N, Al-Daccak R. Manchon E, et al. Int J Mol Sci. 2021 Mar 19;22(6):3130. doi: 10.3390/ijms22063130. Int J Mol Sci. 2021. PMID: 33808520 Free PMC article. Review.
Cited by
- Doping of casted silk fibroin membranes with extracellular vesicles for regenerative therapy: a proof of concept.
Fuest S, Salviano-Silva A, Maire CL, Xu Y, Apel C, Grust ALC, Delle Coste A, Gosau M, Ricklefs FL, Smeets R. Fuest S, et al. Sci Rep. 2024 Feb 12;14(1):3553. doi: 10.1038/s41598-024-54014-y. Sci Rep. 2024. PMID: 38347108 Free PMC article. - Hepatocyte Growth Factor: A Microenvironmental Resource for Leukemic Cell Growth.
Giannoni P, Fais F, Cutrona G, Totero D. Giannoni P, et al. Int J Mol Sci. 2019 Jan 12;20(2):292. doi: 10.3390/ijms20020292. Int J Mol Sci. 2019. PMID: 30642077 Free PMC article. Review. - Recent Advances in RNA Therapy and Its Carriers to Treat the Single-Gene Neurological Disorders.
Lee MJ, Lee I, Wang K. Lee MJ, et al. Biomedicines. 2022 Jan 12;10(1):158. doi: 10.3390/biomedicines10010158. Biomedicines. 2022. PMID: 35052837 Free PMC article. Review. - Mesenchymal stem cell-derived exosomes: A novel and potential remedy for cutaneous wound healing and regeneration.
Hu JC, Zheng CX, Sui BD, Liu WJ, Jin Y. Hu JC, et al. World J Stem Cells. 2022 May 26;14(5):318-329. doi: 10.4252/wjsc.v14.i5.318. World J Stem Cells. 2022. PMID: 35722196 Free PMC article. Review. - Stem cell therapy: old challenges and new solutions.
Balistreri CR, De Falco E, Bordin A, Maslova O, Koliada A, Vaiserman A. Balistreri CR, et al. Mol Biol Rep. 2020 Apr;47(4):3117-3131. doi: 10.1007/s11033-020-05353-2. Epub 2020 Mar 3. Mol Biol Rep. 2020. PMID: 32128709 Review.
References
- Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. - PubMed
- Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–82. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical