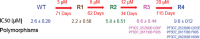Altered Plasmodium falciparum Sensitivity to the Antiretroviral Protease Inhibitor Lopinavir Associated with Polymorphisms in pfmdr1 - PubMed (original) (raw)
Altered Plasmodium falciparum Sensitivity to the Antiretroviral Protease Inhibitor Lopinavir Associated with Polymorphisms in pfmdr1
Ebere Sonoiki et al. Antimicrob Agents Chemother. 2016.
Abstract
The HIV protease inhibitor lopinavir inhibits Plasmodium falciparum aspartic proteases (plasmepsins) and parasite development, and children receiving lopinavir-ritonavir experienced fewer episodes of malaria than those receiving other antiretroviral regimens. Resistance to lopinavir was selected in vitro over ∼9 months, with ∼4-fold decreased sensitivity. Whole-genome sequencing of resistant parasites showed a mutation and increased copy number in pfmdr1 and a mutation in a protein of unknown function, but no polymorphisms in plasmepsin genes.
Keywords: HIV; Plasmodium falciparum; antiretroviral; aspartic protease; drug resistance; drug resistance mechanisms; drug sensitivity; lopinavir; malaria; pfmdr1.
Copyright © 2016 American Society for Microbiology.
Figures
FIG 1
Selection of P. falciparum with decreased sensitivity to lopinavir. Each selection from wild type (WT) to generations R1 to R4 is indicated by an arrow, with the selection concentration and time indicated. Sensitivities of selected strains are shown (50% inhibitory concentration [IC50]; mean of triplicate measures ± standard error of the mean [SEM]). WT sensitivity is the mean of assessments at each time point for cultures grown in parallel without lopinavir. Polymorphisms in R3 and R4 parasites are shown. The copy numbers of PFE1150w were 1 in WT and 4 in R3 and R4 parasites.
Similar articles
- The Glucose Transporter PfHT1 Is an Antimalarial Target of the HIV Protease Inhibitor Lopinavir.
Kraft TE, Armstrong C, Heitmeier MR, Odom AR, Hruz PW. Kraft TE, et al. Antimicrob Agents Chemother. 2015 Oct;59(10):6203-9. doi: 10.1128/AAC.00899-15. Epub 2015 Jul 27. Antimicrob Agents Chemother. 2015. PMID: 26248369 Free PMC article. - Inactivation of Plasmepsins 2 and 3 Sensitizes Plasmodium falciparum to the Antimalarial Drug Piperaquine.
Mukherjee A, Gagnon D, Wirth DF, Richard D. Mukherjee A, et al. Antimicrob Agents Chemother. 2018 Mar 27;62(4):e02309-17. doi: 10.1128/AAC.02309-17. Print 2018 Apr. Antimicrob Agents Chemother. 2018. PMID: 29439977 Free PMC article. - A 2-amino quinoline, 5-(3-(2-(7-chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid, interacts with PfMDR1 and inhibits its drug transport in Plasmodium falciparum.
Edaye S, Reiling SJ, Leimanis ML, Wunderlich J, Rohrbach P, Georges E. Edaye S, et al. Mol Biochem Parasitol. 2014 Jun;195(1):34-42. doi: 10.1016/j.molbiopara.2014.05.006. Epub 2014 Jun 8. Mol Biochem Parasitol. 2014. PMID: 24914817 - Studies on plasmepsins I and II from the malarial parasite Plasmodium falciparum and their exploitation as drug targets.
Moon RP, Bur D, Loetscher H, D'Arcy A, Tyas L, Oefner C, Grueninger-Leitch F, Mona D, Rupp K, Dorn A, Matile H, Certa U, Berry C, Kay J, Ridley RG. Moon RP, et al. Adv Exp Med Biol. 1998;436:397-406. doi: 10.1007/978-1-4615-5373-1_56. Adv Exp Med Biol. 1998. PMID: 9561248 Review. No abstract available. - Antimalarials: Review of Plasmepsins as Drug Targets and HIV Protease Inhibitors Interactions.
Miller Iii WA, Teye J, Achieng AO, Mogire RM, Akala H, Ong'echa JM, Rathi B, Durvasula R, Kempaiah P, Kwofie SK. Miller Iii WA, et al. Curr Top Med Chem. 2019;18(23):2022-2028. doi: 10.2174/1568026619666181130133548. Curr Top Med Chem. 2019. PMID: 30499404 Review.
Cited by
- The natural function of the malaria parasite's chloroquine resistance transporter.
Shafik SH, Cobbold SA, Barkat K, Richards SN, Lancaster NS, Llinás M, Hogg SJ, Summers RL, McConville MJ, Martin RE. Shafik SH, et al. Nat Commun. 2020 Aug 6;11(1):3922. doi: 10.1038/s41467-020-17781-6. Nat Commun. 2020. PMID: 32764664 Free PMC article. - Unrevealing sequence and structural features of novel coronavirus using in silico approaches: The main protease as molecular target.
Ortega JT, Serrano ML, Pujol FH, Rangel HR. Ortega JT, et al. EXCLI J. 2020 Mar 17;19:400-409. doi: 10.17179/excli2020-1189. eCollection 2020. EXCLI J. 2020. PMID: 32210741 Free PMC article. - Advances in omics-based methods to identify novel targets for malaria and other parasitic protozoan infections.
Cowell AN, Winzeler EA. Cowell AN, et al. Genome Med. 2019 Oct 22;11(1):63. doi: 10.1186/s13073-019-0673-3. Genome Med. 2019. PMID: 31640748 Free PMC article. Review. - Plasmid-free CRISPR/Cas9 genome editing in Plasmodium falciparum confirms mutations conferring resistance to the dihydroisoquinolone clinical candidate SJ733.
Crawford ED, Quan J, Horst JA, Ebert D, Wu W, DeRisi JL. Crawford ED, et al. PLoS One. 2017 May 22;12(5):e0178163. doi: 10.1371/journal.pone.0178163. eCollection 2017. PLoS One. 2017. PMID: 28542423 Free PMC article.
References
- World Health Organization. 2015. World malaria report 2015. World Health Organization, Geneva, Switzerland.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources