TRPC3 positively regulates reactive oxygen species driving maladaptive cardiac remodeling - PubMed (original) (raw)
Takuro Numaga-Tomita 1 3, Masahiko Watanabe 4, Takuya Kuroda 5, Akiyuki Nishimura 1 3, Kei Miyano 6, Satoshi Yasuda 5, Koichiro Kuwahara 7, Yoji Sato 2 5, Tomomi Ide 8, Lutz Birnbaumer 9 10, Hideki Sumimoto 6, Yasuo Mori 11, Motohiro Nishida 1 2 3 12
Affiliations
- PMID: 27833156
- PMCID: PMC5105134
- DOI: 10.1038/srep37001
TRPC3 positively regulates reactive oxygen species driving maladaptive cardiac remodeling
Naoyuki Kitajima et al. Sci Rep. 2016.
Abstract
Reactive oxygen species (ROS) produced by NADPH oxidase 2 (Nox2) function as key mediators of mechanotransduction during both physiological adaptation to mechanical load and maladaptive remodeling of the heart. This is despite low levels of cardiac Nox2 expression. The mechanism underlying the transition from adaptation to maladaptation remains obscure, however. We demonstrate that transient receptor potential canonical 3 (TRPC3), a Ca2+-permeable channel, acts as a positive regulator of ROS (PRROS) in cardiomyocytes, and specifically regulates pressure overload-induced maladaptive cardiac remodeling in mice. TRPC3 physically interacts with Nox2 at specific C-terminal sites, thereby protecting Nox2 from proteasome-dependent degradation and amplifying Ca2+-dependent Nox2 activation through TRPC3-mediated background Ca2+ entry. Nox2 also stabilizes TRPC3 proteins to enhance TRPC3 channel activity. Expression of TRPC3 C-terminal polypeptide abolished TRPC3-regulated ROS production by disrupting TRPC3-Nox2 interaction, without affecting TRPC3-mediated Ca2+ influx. The novel TRPC3 function as a PRROS provides a mechanistic explanation for how diastolic Ca2+ influx specifically encodes signals to induce ROS-mediated maladaptive remodeling and offers new therapeutic possibilities.
Figures
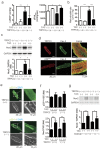
Figure 1. TRPC3 deletion suppresses TAC-induced LV dysfunction and dilation through Nox2 inhibition.
(a) Left ventricular end-diastolic pressure (LVEDP; left) and dP/dTmax (right) in TAC-operated TRPC3(+/+) (n = 13) and TRPC3(−/−) (n = 12) mice 6 week post-operation. (b) Myocardial malondialdehyde concentrations 1 week after TAC (n = 4). (c) Abundance of Nox2 protein in TRPC3(+/+) and TRPC3(−/−) hearts 1 week after TAC (n = 3). (d) Representative immunofluorescence images of TRPC3, p22phox, and caveolin-3 (Cav-3) in adult mouse cardiomyocytes isolated from muscle LIM protein-deficient hearts. (e) Representative immunofluorescence images of p22phox in adult mouse cardiomyocytes: green, anti-p22phox; blue, DAPI. (f) Relative abundances of p22phox and Nox2 mRNA in mouse hearts 1 week after TAC (n = 4). (g) Abundance of Nox2 protein in TRPC6(+/+) and TRPC6(−/−) hearts 1 week after TAC (n = 3). Error bars, s.e.m. *P < 0.05, **P < 0.01.
Figure 2. TRPC3 plays a critical role in Mechanical stretch-induced ROS production.
(a,b) Effects of siRNA targeting TRPC1, C3 or C6 on mechanical stretch (MS)-induced ROS production (n = 3). (c) mRNA expression of either TRPC1 or TRPC6 in NRCM transfected with siRNAs against either TRPC1 or TRPC6, respectively (n = 3). (d,e) Time courses of MS-induced ROS production in NRCMs treated with GsMTx-4 (1 μM; (d) or TRPV4 inhibitor (RN1734, 50 μM; (e) Reagents were added to cells 5 min before MS (n = 3). (f) MS-induced ROS production in TRPC(1–7)-deficient MEF cells expressing TRPC3, TRPC6, TRPC7, or LacZ (n = 30). Data are representative of three independent experiments. (g–i) Effect of TRPC3 siRNA on the protein abundances of TRPC3 (h) and Nox2 (i) protein expressions in NRCMs (n = 3). Error bars, s.e.m. *P < 0.05, **P < 0.01.
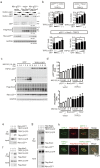
Figure 3. TRPC3 forms a stable ternary complex with Nox2 and p22phox.
(a,b) Expression of Nox2 and p22phox proteins in HEK293 cells that express a different combination of TRPC3-GFP and GFP. Results of a quantitative analysis are shown in (b) (n = 3). (c) Nox2 mRNA amounts in HEK293 cells co-expressing Nox2 with GFP or TRPC3-GFP (n = 3). (d) Increased Nox2 and p22phox protein in HEK293 cells co-expressing pore-dead mutant of TRPC3 (n = 3). (e) Interaction of TRPC3 with Nox2 in HEK293 cells. Immunoprecipitation was performed using an anti-flag antibody. (f) Nox2 protein expression in HEK293 cells expressing TRPC3 alone or co-expressing TRPC3 and TRPC6 (n = 3). Error bars, s.e.m. *P < 0.05, **P < 0.01.
Figure 4. TRPC3 forms a stable ternary complex with Nox2 and p22phox proteins in endogenously p22phox -absent CHO cells.
(a) Expression of Nox2 and p22phox proteins in CHO cells that express a different combination of TRPC3-GFP and GFP. (b) Results of quantitative analysis (n = 3). (c) Expression of Nox2 and p22phox co-expressed with either GFP or TRPC3-GFP in MG132 (10 μM)-treated CHO cells. (d) Graphs depict the relative expression of either Nox2 or p22phox protein to that in non-treated cells. Band intensities were normalized by GAPDH. (e–g) Interaction of TRPC3 with p22phox and Nox2 in CHO cells. (h) Localization of Nox2 in CHO cells co-expressing Nox2 with TRPC3-GFP (or GFP-F). Error bars, s.e.m. *P < 0.05, **P < 0.01.
Figure 5. TRPC3 prevents Nox2 protein from proteasomal degradation.
(a–e) Abundances of Nox2 protein (a,b) and mRNAs of TRPC3 (c), Nox2 (d), and p22phox (e) in NRCM transfected with siRNAs targeting TRPC3 with or without MG132. Cells were treated with siRNAs and MG132 (1 μM) simultaneously (n = 3). (f,g) Effect of siRNA targeting TRPC3 on Nox2 protein abundance in cell surface (Surface) and total lysates (Total) from NRCMs (n = 3). GAPDH was used as an internal control. Error bars, s.e.m. *P < 0.05.
Figure 6. Formation of a TRPC3/Nox2 complex promotes TRPC3 channel activity through stabilization at the plasma membrane.
(a) Effect of Nox2 siRNA on expression of TRPC3 in NRCMs (n = 3). (b) Representative images showing the levels of TRPC3-GFP and GFP expression in HEK293 cells co-expressing p22phox or Nox2 (n = 3). (c) Expression of TRPC3-GFP mRNA in HEK293 cells co-expressing p22phox or Nox2 (n = 3). (d–f) Representative time courses of TRPC3 currents (d) and the current-voltage (I-V) relationships (e) and peak TRPC3 current densities at −60 mV (f) induced by 100 μM carbachol (CCh) in HEK293 cells expressing TRPC3-mCherry alone or with p22phox, Nox2, both p22phox and Nox2, or Nox2 treated with DPI. DPI (0.3 μM) was treated 1 min before CCh stimulation. (g,h) Representative Ca2+ responses in the presence (g) or absence (h) of pyrazole-3 (Pyr3, 1 μM) upon mechanical stretch (MS) application. (i) Peak Ca2+ increases after MS in NRCMs treated with (n = 61) or without Pyr3 (n = 78). (j) Changes of minimal [Ca2+]i before and after MS application. Minimal [Ca2+]i from Ca2+ responses in every 1 min were analyzed and represented as diastolic [Ca2+]i. (k) Schematic images showing phosphorylation of p47phox via TRPC3-PKCβ activation induced by MS in the heart. (l–n) Effects of TRPC3 (l,m) or PKCβ (n); 10 μM Gö6976) inhibitors on p47phox phosphorylation induced by MS in NRCMs (n = 3). (o) MS-induced ROS generation in NRCMs treated with a PKCβ inhibitor (n = 3). (p) Co-immunoprecipitation of TRPC3 with PKCβ, Nox2 and p22phox in mouse hearts 1week after TAC operation (n = 3). Error bars, s.e.m.*P < 0.05, **P < 0.01.
Figure 7. Physical interaction between TRPC3 and Nox2 is critical for stabilization of Nox2.
(a) Schematic illustration of TRPC3 terminal deletion mutants. (b,c) Expression of Nox2 and p22phox co-expressed with TRPC3 deletion mutants in HEK293 cells (n = 3). (d) OAG-induced ROS production in NRCMs expressing Nox2-interacting TRPC3 C-terminal fragment (C3-C fragment) (n = 20–28). (e) Co-immunoprecipitation of TRPC3 with Nox2 in the presence or absence of C3-C fragment. Representative blot from three independent experiments was shown. (f) ATP (100 μM)-induced Ca2+ responses in HEK293 cells expressing TRPC3 with or without C3-C fragment (n = 35–51). Timing of solution exchanges were indicated by horizontal bars above the graph. (g) Model of the regulation of TRPC3-Nox2 stability and induction of LV dysfunction induced by diastolic stretch of cardiomyocytes. Error bars, s.e.m.*P < 0.05, **P < 0.01.
Similar articles
- TRPC6 counteracts TRPC3-Nox2 protein complex leading to attenuation of hyperglycemia-induced heart failure in mice.
Oda S, Numaga-Tomita T, Kitajima N, Toyama T, Harada E, Shimauchi T, Nishimura A, Ishikawa T, Kumagai Y, Birnbaumer L, Nishida M. Oda S, et al. Sci Rep. 2017 Aug 8;7(1):7511. doi: 10.1038/s41598-017-07903-4. Sci Rep. 2017. PMID: 28790356 Free PMC article. - TRPC3 Channels in Cardiac Fibrosis.
Numaga-Tomita T, Oda S, Shimauchi T, Nishimura A, Mangmool S, Nishida M. Numaga-Tomita T, et al. Front Cardiovasc Med. 2017 Sep 7;4:56. doi: 10.3389/fcvm.2017.00056. eCollection 2017. Front Cardiovasc Med. 2017. PMID: 28936433 Free PMC article. Review. - TRPC3-Based Protein Signaling Complex as a Therapeutic Target of Myocardial Atrophy.
Nishiyama K, Tanaka T, Nishimura A, Nishida M. Nishiyama K, et al. Curr Mol Pharmacol. 2021;14(2):123-131. doi: 10.2174/1874467213666200407090121. Curr Mol Pharmacol. 2021. PMID: 32264816 Review. - TRPC3-GEF-H1 axis mediates pressure overload-induced cardiac fibrosis.
Numaga-Tomita T, Kitajima N, Kuroda T, Nishimura A, Miyano K, Yasuda S, Kuwahara K, Sato Y, Ide T, Birnbaumer L, Sumimoto H, Mori Y, Nishida M. Numaga-Tomita T, et al. Sci Rep. 2016 Dec 19;6:39383. doi: 10.1038/srep39383. Sci Rep. 2016. PMID: 27991560 Free PMC article. - TRPC3-Nox2 axis mediates nutritional deficiency-induced cardiomyocyte atrophy.
Sudi SB, Tanaka T, Oda S, Nishiyama K, Nishimura A, Sunggip C, Mangmool S, Numaga-Tomita T, Nishida M. Sudi SB, et al. Sci Rep. 2019 Jul 5;9(1):9785. doi: 10.1038/s41598-019-46252-2. Sci Rep. 2019. PMID: 31278358 Free PMC article.
Cited by
- Myocardial TRPC6-mediated Zn2+ influx induces beneficial positive inotropy through β-adrenoceptors.
Oda S, Nishiyama K, Furumoto Y, Yamaguchi Y, Nishimura A, Tang X, Kato Y, Numaga-Tomita T, Kaneko T, Mangmool S, Kuroda T, Okubo R, Sanbo M, Hirabayashi M, Sato Y, Nakagawa Y, Kuwahara K, Nagata R, Iribe G, Mori Y, Nishida M. Oda S, et al. Nat Commun. 2022 Oct 26;13(1):6374. doi: 10.1038/s41467-022-34194-9. Nat Commun. 2022. PMID: 36289215 Free PMC article. - H-Ferritin is essential for macrophages' capacity to store or detoxify exogenously added iron.
Mesquita G, Silva T, Gomes AC, Oliveira PF, Alves MG, Fernandes R, Almeida AA, Moreira AC, Gomes MS. Mesquita G, et al. Sci Rep. 2020 Feb 20;10(1):3061. doi: 10.1038/s41598-020-59898-0. Sci Rep. 2020. PMID: 32080266 Free PMC article. - Structure of the human lipid-gated cation channel TRPC3.
Fan C, Choi W, Sun W, Du J, Lü W. Fan C, et al. Elife. 2018 May 4;7:e36852. doi: 10.7554/eLife.36852. Elife. 2018. PMID: 29726814 Free PMC article. - Trpc6 Promotes Doxorubicin-Induced Cardiomyopathy in Male Mice With Pleiotropic Differences Between Males and Females.
Norton N, Bruno KA, Di Florio DN, Whelan ER, Hill AR, Morales-Lara AC, Mease AA, Sousou JM, Malavet JA, Dorn LE, Salomon GR, Macomb LP, Khatib S, Anastasiadis ZP, Necela BM, McGuire MM, Giresi PG, Kotha A, Beetler DJ, Weil RM, Landolfo CK, Fairweather D. Norton N, et al. Front Cardiovasc Med. 2022 Jan 13;8:757784. doi: 10.3389/fcvm.2021.757784. eCollection 2021. Front Cardiovasc Med. 2022. PMID: 35096991 Free PMC article. - BNIP3L promotes cardiac fibrosis in cardiac fibroblasts through [Ca2+]i-TGF-β-Smad2/3 pathway.
Liu W, Wang X, Mei Z, Gong J, Huang L, Gao X, Zhao Y, Ma J, Qian L. Liu W, et al. Sci Rep. 2017 May 15;7(1):1906. doi: 10.1038/s41598-017-01936-5. Sci Rep. 2017. PMID: 28507335 Free PMC article.
References
- Leask A. Potential therapeutic targets for cardiac fibrosis: TGFβ, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ. res. 106, 1675–1680 (2010). - PubMed
- Prosser B. L., Ward C. W. & Lederer W. J. X-ROS signaling: rapid mechano-chemo transduction in heart. Science 333, 1440–1445 (2011). - PubMed
- Patel V. B. et al.. Loss of p47phox subunit enhances susceptibility to biomechanical stress and heart failure because of dysregulation of cortactin and actin filaments. Circ. res. 112, 1542–1556 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous