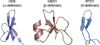Defensins, lectins, mucins, and secretory immunoglobulin A: microbe-binding biomolecules that contribute to mucosal immunity in the human gut - PubMed (original) (raw)
Review
Defensins, lectins, mucins, and secretory immunoglobulin A: microbe-binding biomolecules that contribute to mucosal immunity in the human gut
Phoom Chairatana et al. Crit Rev Biochem Mol Biol. 2017 Feb.
Abstract
In the intestine, the mucosal immune system plays essential roles in maintaining homeostasis between the host and microorganisms, and protecting the host from pathogenic invaders. Epithelial cells produce and release a variety of biomolecules into the mucosa and lumen that contribute to immunity. In this review, we focus on a subset of these remarkable host-defense factors - enteric α-defensins, select lectins, mucins, and secretory immunoglobulin A - that have the capacity to bind microbes and thereby contribute to barrier function in the human gut. We provide an overview of the intestinal epithelium, describe specialized secretory cells named Paneth cells, and summarize our current understanding of the biophysical and functional properties of these select microbe-binding biomolecules. We intend for this compilation to complement prior reviews on intestinal host-defense factors, highlight recent advances in the field, and motivate investigations that further illuminate molecular mechanisms as well as the interplay between these molecules and microbes.
Keywords: Mucosal immunity; Paneth cells; defensins; host defense; lectins; mucins; sIgA.
Figures
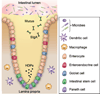
Figure 1
The physical and chemical barriers in a crypt of small intestine. Intestinal epithelial cells form physical and chemical barriers that segregate the luminal microbial community and the mucosal immune system. HDP = host-defense peptide; sIgA = secretory immunoglobulin A. A color version of the figure is available online.
Figure 2
Primary sequences and X-ray crystal structures of select defensins. HD6 (PDB 1ZMQ (Szyk et al., 2006)), HBD1 (PDB 1IJV (Hoover et al., 2001)), and RTD1 (PDB 1HVZ (Trabi et al., 2001)) are representatives of α-, β-, and θ-defensin, respectively. The disulfide linkages are shown in yellow. A color version of the figure is available online.
Figure 3
Defensins are expressed as prepropeptides and exhibit several conserved features. (A) A cartoon represents a prepropeptide. The black arrow indicates the position where a protease cleaves and releases a mature defensin. (B) Amino acid sequences of mature α-defensins in which conserved features are highlighted: cysteine residues and regiospecific disulfide linkages are shown in red, a salt bridge between Arg and Glu residues is shown in blue, a Gly residue in a GXC motif is shown green. A color version of the figure is available online.
Figure 4
Overview of HD5. (A) The amino acid sequence of HD5 and a cartoon showing its secondary structure. The solid red lines represent the disulfide linkages. (B) Crystal structures of HD5 monomer (left) and dimer (right) (PDB 1ZMP (Szyk et al., 2006)). The hydrophobic residues of HD5 are distributed at the tetrameric interface, whereas the Arg residues are distributed on the opposite face of the dimer, which is exposed to the surface after tetramerization. The hydrophobic residues are shown in orange and the Arg residues are shown in pink. A color version of the figure is available online.
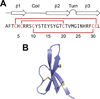
Figure 5
Overview of HD6. (A) The amino acid sequence of HD6 and a cartoon showing its secondary structure. The solid red lines represent the disulfide linkages. (B) Crystal structure of HD6 monomer (PDB 1ZMQ (Szyk et al., 2006)) with disulfide bonds shown in yellow. A color version of the figure is available online.
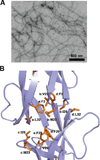
Figure 6
Self-assembly of HD6. (A) Transmission electron micrograph of 20 µM HD6 in 10 mM sodium phosphate, pH 7.4. (B) Crystal structure of HD6 monomers (PDB 1ZMQ (Szyk et al., 2006)) showing the hydrophobic pocket that drives the self-assembly of HD6. Individual HD6 monomers are labeled as a–d. A color version of the figure is available online.
Figure 7
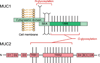
Structures of mucins. The structure of mucin 1 (MUC1) represents the typical structure of membrane-bound mucins in the GI tract. The extracellular TRR is heavily _O_-glycosylated, and the protein is _N_-glycosylated near the SEA domain. The cytoplasmic domain of MUC1 is involved in intracellular transduction. Mucin 2 (MUC2) is a major component of the secreted mucus barrier in the intestine. The TRRs are heavily _O_-glycosylated and the N- and C-terminal D domains are involved in homo-oligomerization. A color version of the figure is available online.
Figure 8
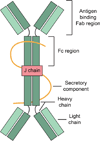
A cartoon represents the overview structure of sIgA. SIgA are dimeric with two IgA molecules held together by a joining chain (J-chain). Each IgA molecule consists of two heavy chains and two light chains. The secretory component protects the antibody from being degraded by the enzymes of the digestive system. A color version of the figure is available online.
Similar articles
- Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa.
Salzman NH, Underwood MA, Bevins CL. Salzman NH, et al. Semin Immunol. 2007 Apr;19(2):70-83. doi: 10.1016/j.smim.2007.04.002. Epub 2007 May 7. Semin Immunol. 2007. PMID: 17485224 Review. - Paneth cell defensins: key effector molecules of innate immunity.
Bevins CL. Bevins CL. Biochem Soc Trans. 2006 Apr;34(Pt 2):263-6. doi: 10.1042/BST20060263. Biochem Soc Trans. 2006. PMID: 16545089 Review. - Enteric α-defensins on the verge of intestinal immune tolerance and inflammation.
Filipp D, Brabec T, Vobořil M, Dobeš J. Filipp D, et al. Semin Cell Dev Biol. 2019 Apr;88:138-146. doi: 10.1016/j.semcdb.2018.01.007. Epub 2018 Feb 1. Semin Cell Dev Biol. 2019. PMID: 29355606 Review. - Events at the host-microbial interface of the gastrointestinal tract. V. Paneth cell alpha-defensins in intestinal host defense.
Bevins CL. Bevins CL. Am J Physiol Gastrointest Liver Physiol. 2005 Aug;289(2):G173-6. doi: 10.1152/ajpgi.00079.2005. Am J Physiol Gastrointest Liver Physiol. 2005. PMID: 16014978 Review. - Human α-Defensin 6: A Small Peptide That Self-Assembles and Protects the Host by Entangling Microbes.
Chairatana P, Nolan EM. Chairatana P, et al. Acc Chem Res. 2017 Apr 18;50(4):960-967. doi: 10.1021/acs.accounts.6b00653. Epub 2017 Mar 15. Acc Chem Res. 2017. PMID: 28296382 Free PMC article. Review.
Cited by
- Alleviating effect of chlorogenic acid on oxidative damage caused by hydrogen peroxide in bovine intestinal epithelial cells.
Lu J, An Y, Wang X, Zhang C, Guo S, Ma Y, Qiu Y, Wang S. Lu J, et al. J Vet Med Sci. 2024 Sep 20;86(9):1016-1026. doi: 10.1292/jvms.24-0148. Epub 2024 Jul 29. J Vet Med Sci. 2024. PMID: 39069486 Free PMC article. - Loss of gut barrier integrity triggers activation of islet-reactive T cells and autoimmune diabetes.
Sorini C, Cosorich I, Lo Conte M, De Giorgi L, Facciotti F, Lucianò R, Rocchi M, Ferrarese R, Sanvito F, Canducci F, Falcone M. Sorini C, et al. Proc Natl Acad Sci U S A. 2019 Jul 23;116(30):15140-15149. doi: 10.1073/pnas.1814558116. Epub 2019 Jun 10. Proc Natl Acad Sci U S A. 2019. PMID: 31182588 Free PMC article. - Yeast fermentate prebiotic improves intestinal barrier integrity during heat stress by modulation of the gut microbiota in rats.
Ducray HAG, Globa L, Pustovyy O, Morrison E, Vodyanoy V, Sorokulova I. Ducray HAG, et al. J Appl Microbiol. 2019 Oct;127(4):1192-1206. doi: 10.1111/jam.14361. Epub 2019 Jul 9. J Appl Microbiol. 2019. PMID: 31230390 Free PMC article. - Reg4 and complement factor D prevent the overgrowth of E. coli in the mouse gut.
Qi H, Wei J, Gao Y, Yang Y, Li Y, Zhu H, Su L, Su X, Zhang Y, Yang R. Qi H, et al. Commun Biol. 2020 Sep 2;3(1):483. doi: 10.1038/s42003-020-01219-2. Commun Biol. 2020. PMID: 32879431 Free PMC article. - Key Stress Response Mechanisms of Probiotics During Their Journey Through the Digestive System: A Review.
Castro-López C, Romero-Luna HE, García HS, Vallejo-Cordoba B, González-Córdova AF, Hernández-Mendoza A. Castro-López C, et al. Probiotics Antimicrob Proteins. 2023 Oct;15(5):1250-1270. doi: 10.1007/s12602-022-09981-x. Epub 2022 Aug 24. Probiotics Antimicrob Proteins. 2023. PMID: 36001271 Review.
References
- Asker N, Axelsson MAB, Olofsson S-O, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J. Biol. Chem. 1998;273:18857–18863. - PubMed
- Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, Ouellette AJ. Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000;1:113–118. - PubMed
- Bevins CL, Salzman NH. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat. Rev. Microbiol. 2011;9:356–368. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources