Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder - PubMed (original) (raw)
Review
Convergence of Sex Differences and the Neuroimmune System in Autism Spectrum Disorder
Margaret M McCarthy et al. Biol Psychiatry. 2017.
Abstract
The male bias in autism spectrum disorder incidence is among the most extreme of all neuropsychiatric disorders, yet the origins of the sex difference remain obscure. Developmentally, males are exposed to high levels of testosterone and its byproduct, estradiol. Together these steroids modify the course of brain development by altering neurogenesis, cell death, migration, differentiation, dendritic and axonal growth, synaptogenesis, and synaptic pruning, all of which can be deleteriously impacted during the course of developmental neuropsychiatric disorders. Elucidating the cellular mechanisms by which steroids modulate brain development provides valuable insights into how these processes may go awry. An emerging theme is the role of inflammatory signaling molecules and the innate immune system in directing brain masculinization, the evidence for which we review here. Evidence is also emerging that the neuroimmune system is overactivated in individuals with autism spectrum disorder. These combined observations lead us to propose that the natural process of brain masculinization puts males at risk by moving them closer to a vulnerability threshold that could more easily be breached by inflammation during critical periods of brain development. Two brain regions are highlighted: the preoptic area and the cerebellum. Both are developmentally regulated by the inflammatory prostaglandin E2, but in different ways. Microglia, innate immune cells of the brain, and astrocytes are also critical contributors to masculinization and illustrate the importance of nonneuronal cells to the health of the developing brain.
Keywords: Androgens; Autism spectrum disorder; Cerebellum; Estrogens; Masculinization; Microglia; Preoptic area; Prostaglandins.
Copyright © 2016 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Figures
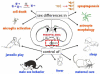
Figure 1. Masculinization of the brain converges with inflammation and enhances male vulnerability to ASD
Feminization and masculinization are distinct developmental processes. In rodents normal masculinization of some brain regions involves inflammatory signaling molecules such as prostaglandins which are derived from activated innate immune cells, the microglia, as well as reactive astrocytes. Inflammation during pregnancy in humans is a putative risk factor for development of ASD, with evidence that the greater the inflammation the more severe the disorder(20). Whether in utero inflammation increases the risk for ASD disproportionately in males is unknown. Based on research in rodents and correlational evidence in humans(37) we propose that females have very low levels of inflammatory signaling in the brain (green) while the natural process of masculinization increases inflammation in males (yellow-orange) and pushes males closer to a threshold of vulnerability which can be more easily breached if inflammation occurs during a developmental sensitive period (orange-red).
Figure 2. The preoptic area is sexually dimorphic and impacts multiple behaviors with relevance to ASD
The POA (red box) is a small but complex brain region that contains the medial preoptic nucleus (MPN) and hosts multiple neuroanatomical sex differences and is central to the control of motivated, affiliative and nurturing behaviors as well as sleep and fever generation, all of which are central to the phenotype of ASD. The POA is also the embryonic source of GABA neurons to the amygdala, hippocampus and prefrontal cortex and shares reciprocal connections with components of the reward pathway and those areas regulating fear and social behaviors.
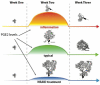
Figure 3. Purkinje neuron development is regulated by PGE2 during a sensitive window
The principle neurons of the cerebellum show dramatic growth and differentiation during the first 3 postnatal weeks of life in the laboratory rat. Endogenous PGE2 is elevated during the 2nd postnatal week and is a critical regulator of normal Purkinje neuron growth. If PGE2 production is increased by inflammation during that week, the Purkinje neuron growth is stunted. If PGE2 production is decreased by treatment with NSAID inhibitors of cyclooxygenase, the Purkinje neurons show excessive growth of the dendritic tree. Changes in PGE2 levels in either the 1st or 3rd postnatal week has no impact on Purkinje neuron growth and this appears to be due to an intrinsic gene expression program in which the transducers of the PGE2 signal are up regulated during the 2nd week but expressed only at low levels during the 1st and 3rd weeks. Thus there is a narrowly defined sensitive window during which inflammation or treatment with NSAIDs dysregulates cerebellar development.
Figure 4. Different sensitive periods at different life stages of rats and humans
The developmental profile of the rat is shifted from humans in that a newborn pup is roughly equivalent to a mid- to late-gestation human fetus. The sensitive period for sexual differentiation of the preoptic area in the rat is operationally defined by the onset of testicular androgen production in male fetuses on embryonic day 18 and the loss of sensitivity of females to exogenous hormone treatment by the end of the first postnatal week. In humans the sensitive period for the preoptic area begins during the 2nd trimester with fetal androgen production and probably ends prior to birth although this conclusion is constrained by a lack of experimental data. The sensitive period we have identified in cerebellar development is during the second postnatal week in the rat which corresponds to the peripartum period in the human. The factors constraining the sensitive period in the rat are the onset and offset of gene expression profiles. Whether a similar profile exists in humans is currently unknown.
Similar articles
- Prenatal Allergen Exposure Perturbs Sexual Differentiation and Programs Lifelong Changes in Adult Social and Sexual Behavior.
Lenz KM, Pickett LA, Wright CL, Galan A, McCarthy MM. Lenz KM, et al. Sci Rep. 2019 Mar 18;9(1):4837. doi: 10.1038/s41598-019-41258-2. Sci Rep. 2019. PMID: 30886382 Free PMC article. - Microglia are essential to masculinization of brain and behavior.
Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Lenz KM, et al. J Neurosci. 2013 Feb 13;33(7):2761-72. doi: 10.1523/JNEUROSCI.1268-12.2013. J Neurosci. 2013. PMID: 23407936 Free PMC article. - The influence of neuroinflammation in Autism Spectrum Disorder.
Matta SM, Hill-Yardin EL, Crack PJ. Matta SM, et al. Brain Behav Immun. 2019 Jul;79:75-90. doi: 10.1016/j.bbi.2019.04.037. Epub 2019 Apr 25. Brain Behav Immun. 2019. PMID: 31029798 Review. - Sex differences in neuroimmunity as an inherent risk factor.
McCarthy MM. McCarthy MM. Neuropsychopharmacology. 2019 Jan;44(1):38-44. doi: 10.1038/s41386-018-0138-1. Epub 2018 Jun 29. Neuropsychopharmacology. 2019. PMID: 29977075 Free PMC article. Review. - Role of Microglia in Autism: Recent Advances.
Takano T. Takano T. Dev Neurosci. 2015;37(3):195-202. doi: 10.1159/000398791. Epub 2015 May 21. Dev Neurosci. 2015. PMID: 25998072 Review.
Cited by
- Reproducible Sex Differences in Personalized Functional Network Topography in Youth.
Keller AS, Sun KY, Francisco A, Robinson H, Beydler E, Bassett DS, Cieslak M, Cui Z, Davatzikos C, Fan Y, Gardner M, Kishton R, Kornfield SL, Larsen B, Li H, Linder I, Pines A, Pritschet L, Raznahan A, Roalf DR, Seidlitz J, Shafiei G, Shinohara RT, Wolf DH, Alexander-Bloch A, Satterthwaite TD, Shanmugan S. Keller AS, et al. bioRxiv [Preprint]. 2024 Sep 29:2024.09.26.615061. doi: 10.1101/2024.09.26.615061. bioRxiv. 2024. PMID: 39386637 Free PMC article. Preprint. - Sex-Differential Gene Expression in Developing Human Cortex and Its Intersection With Autism Risk Pathways.
Kissel LT, Pochareddy S, An JY, Sestan N, Sanders SJ, Wang X, Werling DM. Kissel LT, et al. Biol Psychiatry Glob Open Sci. 2024 Apr 26;4(4):100321. doi: 10.1016/j.bpsgos.2024.100321. eCollection 2024 Jul. Biol Psychiatry Glob Open Sci. 2024. PMID: 38957312 Free PMC article. - Gestational hypothyroxinemia induces ASD-like phenotypes in behavior, proinflammatory markers, and glutamatergic protein expression in mouse offspring of both sexes.
González-Madrid E, Rangel-Ramírez MA, Opazo MC, Méndez L, Bohmwald K, Bueno SM, González PA, Kalergis AM, Riedel CA. González-Madrid E, et al. Front Endocrinol (Lausanne). 2024 May 1;15:1381180. doi: 10.3389/fendo.2024.1381180. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38752179 Free PMC article. - PLASTAMINATION: Outcomes on the Central Nervous System and Reproduction.
Santoro A, Marino M, Vandenberg LN, Szychlinska MA, Lamparelli EP, Scalia F, Rocca ND, D'Auria R, Giovanna Pastorino GM, Porta GD, Operto FF, Viggiano A, Cappello F, Meccariello R. Santoro A, et al. Curr Neuropharmacol. 2024;22(11):1870-1898. doi: 10.2174/1570159X22666240216085947. Curr Neuropharmacol. 2024. PMID: 38549522 Free PMC article. Review. - Role of estrogen in sex differences in memory, emotion and neuropsychiatric disorders.
Iqbal J, Huang GD, Xue YX, Yang M, Jia XJ. Iqbal J, et al. Mol Biol Rep. 2024 Mar 12;51(1):415. doi: 10.1007/s11033-024-09374-z. Mol Biol Rep. 2024. PMID: 38472517 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical