Intermetallic compounds in heterogeneous catalysis-a quickly developing field - PubMed (original) (raw)
Intermetallic compounds in heterogeneous catalysis-a quickly developing field
Marc Armbrüster et al. Sci Technol Adv Mater. 2014.
Abstract
The application of intermetallic compounds for understanding in heterogeneous catalysis developed in an excellent way during the last decade. This review provides an overview of concepts and developments revealing the potential of intermetallic compounds in fundamental as well as applied catalysis research. Intermetallic compounds may be considered as platform materials to address current and future catalytic challenges, e.g. in respect to the energy transition.
Keywords: acetylene semi-hydrogenation; complex metallic alloy; heterogeneous catalysis; intermetallic compound; methanol steam reforming; selective hydrogenation.
Figures
Figure 1
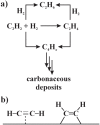
Reaction network of the semi-hydrogenation of acetylene (a); _π_- and di-_σ_-bonded acetylene (b).
Figure 2
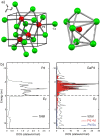
Unit cell of GaPd and coordination of palladium in GaPd (a). Density of states for elemental palladium as well as for GaPd (b).
Figure 3
Ga–Pd phase diagram [32, 33] (top) and coordination of the palladium atoms in intermetallic Ga–Pd compounds as well as the alloy Ga5Pd95 and elemental palladium (bottom, gallium atoms are blue, palladium atoms are red; mixed occupancy in the alloy is indicated by purple).
Figure 4
Electron localization function in Ga7Pd3, electron localizability indicator in GaPd and GaPd2 (from top) revealing covalent contributions to the chemical bonding in all compounds.
Figure 5
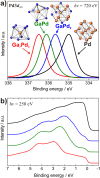
XPS spectra of the intermetallic compounds in comparison to elemental palladium: Pd3d5/2 core level spectra (a) and valence band spectra (b) (identical color code in both panels).
Figure 6
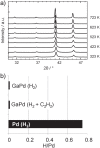
Temperature-dependent powder x-ray diffraction of GaPd in 50% H2 in helium (a). Results from PGAA for GaPd in pure hydrogen as well as under reactive conditions in comparison to elemental palladium (b).
Figure 7
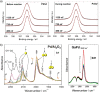
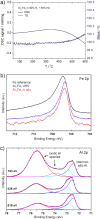
(a) XPS spectra of the Pd 3d5/2 region of GaPd in UHV (left) and reactive atmosphere (400 K) (right). (b) Infrared spectra of CO adsorpt on a commercial 5% Pd/Al2O3 (left, arrows indicate falling partial pressure) and unsupported GaPd powder (right, the arrow indicates increasing partial pressure) revealing only isolated on-top adsorption on GaPd.
Figure 8
Conversion of acetylene (a) and selectivity to ethylene (b) for the intermetallic compounds Ga7Pd3, GaPd and GaPd2 in comparison to 5% Pd/Al2O3 and an unsupported Ga5Pd95 alloy (identical color code in both panels).
Figure 9
Crystal structure of Al13Fe4 highlighting the Fe–Al–Fe groups and their surroundings (a). Electronic density of states of Al13Fe4 (b).
Figure 10
DSC/TG of Al13Fe4 powder in 50% H2/He (a), XPS of the single-crystalline (010) surface: (b) Fe 2p in UHV and in situ in comparison to elemental iron foil and (c) depth profile of the Al 2p region corresponding to inelastic mean free paths of 6.6, 11.3 and 14.7 nm (top to bottom).
Figure 11
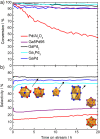
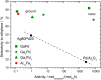
Comparison of several intermetallic catalysts to supported 5% Pd/Al2O3 and the unsupported substitutional alloy Ag80Pd20. The dashed line is a guide to the eye.
Figure 12
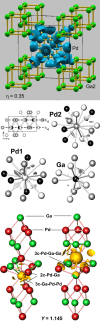
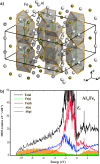
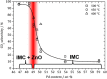
Composition dependent CO2 selectivity in MSR at different temperatures over unsupported ZnPd. Results from in situ ambient pressure XPS measurements are summarized at the bottom (IMC: intermetallic compound), revealing the presence of ZnO in the case of samples rich in Zn.
Figure 13
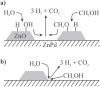
Two pathways for methanol steam reforming: the ZnPd/ZnO interface holds the active sites (a) or the reaction proceeds via spill-over of activated species from ZnPd and/or ZnO (b).
Figure 14
Different leaching behaviors of the quasicrystalline compound Al63Cu25Fe12 and the crystalline compound Al70Cu20Fe10, leading to different Cu morphologies after leaching (adapted with permission from [100]).
Similar articles
- Intermetallic compounds in catalysis - a versatile class of materials meets interesting challenges.
Armbrüster M. Armbrüster M. Sci Technol Adv Mater. 2020 Jun 15;21(1):303-322. doi: 10.1080/14686996.2020.1758544. Sci Technol Adv Mater. 2020. PMID: 33628119 Free PMC article. Review. - Properties of Bulk In-Pt Intermetallic Compounds in Methanol Steam Reforming.
Köwitsch N, Barth S, Ploner K, Blume R, Teschner D, Penner S, Armbrüster M. Köwitsch N, et al. Chemphyschem. 2022 Apr 20;23(8):e202200074. doi: 10.1002/cphc.202200074. Epub 2022 Mar 21. Chemphyschem. 2022. PMID: 35312211 Free PMC article. - Steering the Catalytic Properties of Intermetallic Compounds and Alloys in Reforming Reactions by Controlled in Situ Decomposition and Self-Activation.
Penner S, Kheyrollahi Nezhad PD. Penner S, et al. ACS Catal. 2021 May 7;11(9):5271-5286. doi: 10.1021/acscatal.1c00718. Epub 2021 Apr 16. ACS Catal. 2021. PMID: 34055455 Free PMC article. Review. - Kinetic Parameters for the Selective Hydrogenation of Acetylene on GaPd2 and GaPd.
Zimmermann RR, Hahn T, Reschetilowski W, Armbrüster M. Zimmermann RR, et al. Chemphyschem. 2017 Sep 20;18(18):2517-2525. doi: 10.1002/cphc.201700535. Epub 2017 Aug 16. Chemphyschem. 2017. PMID: 28815973 - Selective hydrogenation of acetylene on Cu-Pd intermetallic compounds and Pd atoms substituted Cu(111) surfaces.
Yuan D , Cai L , Xie T , Liao H , Hu W . Yuan D , et al. Phys Chem Chem Phys. 2021 Apr 14;23(14):8653-8660. doi: 10.1039/d0cp05285j. Epub 2021 Mar 29. Phys Chem Chem Phys. 2021. PMID: 33876026
Cited by
- Free-atom-like d states beyond the dilute limit of single-atom alloys.
Rosen AS, Vijay S, Persson KA. Rosen AS, et al. Chem Sci. 2023 Jan 19;14(6):1503-1511. doi: 10.1039/d2sc05772g. eCollection 2023 Feb 8. Chem Sci. 2023. PMID: 36794204 Free PMC article. - Extraordinarily large kinetic isotope effect on alkene hydrogenation over Rh-based intermetallic compounds.
Furukawa S, Yi P, Kunisada Y, Shimizu KI. Furukawa S, et al. Sci Technol Adv Mater. 2019 Jul 11;20(1):805-812. doi: 10.1080/14686996.2019.1642139. eCollection 2019. Sci Technol Adv Mater. 2019. PMID: 31489053 Free PMC article. - Ca-Ag compounds in ethylene epoxidation reaction.
Antonyshyn I, Sichevych O, Ormeci A, Burkhardt U, Rasim K, Titlbach S, Armbrüster M, Schunk SA, Grin Y. Antonyshyn I, et al. Sci Technol Adv Mater. 2019 Aug 14;20(1):902-916. doi: 10.1080/14686996.2019.1655664. eCollection 2019. Sci Technol Adv Mater. 2019. PMID: 31579432 Free PMC article. - Design of Single-Atom Catalysts and Tracking Their Fate Using Operando and Advanced X-ray Spectroscopic Tools.
Sarma BB, Maurer F, Doronkin DE, Grunwaldt JD. Sarma BB, et al. Chem Rev. 2023 Jan 11;123(1):379-444. doi: 10.1021/acs.chemrev.2c00495. Epub 2022 Nov 23. Chem Rev. 2023. PMID: 36418229 Free PMC article. Review. - Interplay of Atomic Interactions in the Intermetallic Semiconductor Be5 Pt.
Amon A, Svanidze E, Ormeci A, König M, Kasinathan D, Takegami D, Prots Y, Liao YF, Tsuei KD, Tjeng LH, Leithe-Jasper A, Grin Y. Amon A, et al. Angew Chem Int Ed Engl. 2019 Oct 28;58(44):15928-15933. doi: 10.1002/anie.201909782. Epub 2019 Sep 24. Angew Chem Int Ed Engl. 2019. PMID: 31483920 Free PMC article. Review.
References
- Grin Y. In: Comprehensive Inorganic Chemistry II. Reedijk J, and Poeppelmeier K, , editors. vol 2. Oxford: Elsevier; 2013. pp. 359–73.
- Nolas G S, Cohn J L, Slack G A. and Schujman S B. Appl. Phys. Lett. 1998;73:178. doi: 10.1063/1.121747. - DOI
- Menth A, Nagel H. and Perkins R S. Ann. Rev. Mater. Sci. 1978;8:21. doi: 10.1146/annurev.ms.08.080178.000321. - DOI
- Kohlmann H. Encyclopedia of Physical Science and Technology. vol 9. San Diego, CA: Academic; 2002. pp. 441–58.
LinkOut - more resources
Full Text Sources
Other Literature Sources