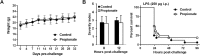Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo - PubMed (original) (raw)
Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo
Eleonora Ciarlo et al. Sci Rep. 2016.
Abstract
Short chain fatty acids (SCFAs) produced by intestinal microbes mediate anti-inflammatory effects, but whether they impact on antimicrobial host defenses remains largely unknown. This is of particular concern in light of the attractiveness of developing SCFA-mediated therapies and considering that SCFAs work as inhibitors of histone deacetylases which are known to interfere with host defenses. Here we show that propionate, one of the main SCFAs, dampens the response of innate immune cells to microbial stimulation, inhibiting cytokine and NO production by mouse or human monocytes/macrophages, splenocytes, whole blood and, less efficiently, dendritic cells. In proof of concept studies, propionate neither improved nor worsened morbidity and mortality parameters in models of endotoxemia and infections induced by gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae), gram-positive bacteria (Staphylococcus aureus, Streptococcus pneumoniae) and Candida albicans. Moreover, propionate did not impair the efficacy of passive immunization and natural immunization. Therefore, propionate has no significant impact on host susceptibility to infections and the establishment of protective anti-bacterial responses. These data support the safety of propionate-based therapies, either via direct supplementation or via the diet/microbiota, to treat non-infectious inflammation-related disorders, without increasing the risk of infection.
Figures
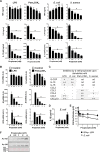
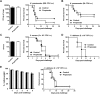
Figure 1. Impact of propionate on the response of macrophages to microbial stimulation.
BMDMs were pre-incubated for 1 h with increasing concentrations (0, 0.06, 0.12, 0.25, 0.5, 1, 2 and 4 mM) of propionate before exposure for 4, 8 or 24 h to LPS (10 ng/ml), Pam3CSK4 (10 ng/ml), E. coli (106 CFU/ml), S. aureus (107 CFU/ml) or a combination of IFNγ (100 U/ml) plus LPS (10 ng/ml). (A,B) TNF, IL-6 and IL-12p40 concentrations in cell culture supernatants and Tnf, Il6, Il12b mRNA levels were quantified by ELISA (A, t = 8 h) and real time-PCR (B, t = 4 h). No cytokine was detected in the supernatants of unstimulated cells (P < 0.001 vs stimulus alone). _Tnf, Il6_ and _Il12b_ mRNA levels were normalized to _Hprt_ mRNA levels. Data are means ± SD of triplicate samples from one experiment performed with 4 mice and representative of 2 experiments. *_P_ < 0.05 vs stimulus without propionate. A.U.: arbitrary units. (**C**) The production of G-CSF, IL-10, IL-18, CCL2, CCL3, CCL4, CCL5 and CXCL10 was assessed by the Luminex technology (t = 8 h). Data summarize the impact of 2 mM propionate on mediators produced in response to LPS, _E. coli_, Pam3CSK4 and _S. aureus_: −, no inhibition; +, 1.5-2-fold inhibition; ++, >2-fold inhibition. Quantification is from one experiment performed with 4 mice. (D) IL-1β in cell culture supernatants. Data are means ± SD of triplicate samples from one experiment performed with 2 mice. *P < 0.05 vs no propionate. (E) Nitrites/nitrates were quantified using the Griess reagent (t = 24 h). Data are means ± SD of quadruplicate samples from one experiment performed with 4 mice. *P < 0.05 when comparing propionate at all concentrations vs no propionate. (F) Western blot analysis of acetylated histone 3 (Ac-H3) and Ac-H4 in BMDMs treated for 18 h with propionate. Ponceau staining of the membrane shows equal loading of total histones. Full-length blots are presented in Supplementary Figure S1.
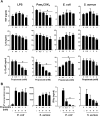
Figure 2. Impact of propionate on the response of dendritic cells and splenocytes.
(A) BMDCs were pre-incubated for 1 h with increasing concentrations (0, 0.5, 1, 2 and 4 mM) of propionate before exposure for 8 h to LPS (10 ng/ml), Pam3CSK4 (10 ng/ml), E. coli (106 CFU/ml) and S. aureus (107 CFU/ml). TNF, IL-6 and IL-12p40 concentrations in cell culture supernatants were quantified by ELISA. Data are means ± SD of triplicate samples from one experiment performed with 4 mice and representative of 2 experiments. No cytokine was detected in the supernatants of unstimulated cells (P < 0.001 vs stimulus alone). (B) Mouse splenocytes were incubated for 48 h with or without propionate and E. coli or S. aureus (106 CFU/ml). Proliferation was measured by 3H-thymidine incorporation. IFNγ concentrations in cell culture supernatants were quantified by ELISA. Data are means ± SD of triplicate samples from one experiment performed with 4 mice. *P < 0.05 vs stimulus without propionate.
Figure 3. Impact of propionate on the response of human whole blood and monocytes.
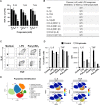
Whole blood from 3 healthy subjects was incubated for 18 h with propionate and LPS (100 ng/ml). (A) TNF released by whole blood collected at 8 am, 1 pm and 7 pm was quantified by ELISA. Data are expressed as the percentage of maximal (LPS without propionate) TNF release. No TNF was detected in the absence of LPS stimulation (not shown). Data are means ± SD from 3 healthy subjects. P < 0.005 when comparing 0.2 and 2 mM propionate with 0 mM propionate. (B) TNF, IL-1β, IL-1RA, IL-10, IL-12p40, CCL2, CCL3, CCL4, CXCL8 and CXCL10 were quantified by Luminex. Results summarize the number of donors in whom propionate inhibited significantly (P < 0.05) and by at least 2-fold cytokine release. (C,D) PBMCs were incubated for 1 h with 2 mM propionate and stimulated for 4 h with LPS (100 ng/ml) and Pam3CSK4 (1 μg/ml). TNF and IL-6 expression in CD14+ monocytes was analyzed by flow cytometry to calculate the percentage of positive cells (C) and mean fluorescence intensity (MFI) (D). Data are means ± SD from one experiment performed with 2 donors. (E) Whole blood incubated for 4 h with 2 mM propionate and 100 ng/ml LPS was fixed with Smart Tube stabilizer, and processed by CyTOF as described in Materials and Methods. Left: t-SNE scatter plot of non-granulocyte events. Right: t-SNE plot with arcsinh transformed signal intensity of IL-6 and TNF. Data are representative of results obtained with 3 donors.
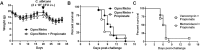
Figure 4. Propionate does not protect from lethal endotoxemia.
BALB/c mice (n = 16 per group) were treated with or without 200 mM propionate in drinking water for 1 month. (A) Weight of animals under propionate treatment. (B) Severity scores (P > 0.1) and survival (P = 0.3) of mice challenged with LPS (250 μg i.p.).
Figure 5. Propionate does not protect from lethal sepsis.
BALB/c mice were treated with or without 200 mM propionate in drinking water for 3 weeks. (A,B) Bacterial counts in lungs 48 h post-infection and survival of mice (n = 10 per group) challenged i.n. with 200 CFU (A) or 20 CFU (B) of K. pneumoniae. P = 0.4, 0.8 and 0.7, respectively. (C) Bacterial counts in blood 24 h post-infection and survival of mice (n = 15 per group) challenged with S. aureus (2 × 107 CFU i.v.). P = 0.9 and P = 0.6. (D,E) Survival and body weight of mice (n = 8 per group) challenged with C. albicans (5 × 105 CFU i.v. in D and 2 × 105 CFU i.v. in E). P = 0.1, P > 0.1 and P = 0.8, respectively.
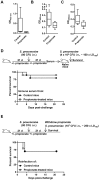
Figure 6. Propionate does not protect from candidiasis mice depleted of gut microbiota.
BALB/c mice (n = 10 per group) were treated with ciprofloxacin (0.2 mg/ml) and metronidazole (1 mg/ml) or metronidazole or vancomycin (1 mg/ml) with or without 200 mM propionate in drinking water for 3 weeks and challenged with C. albicans (2 × 105 CFU i.v.). (A) Body weight. (B,C) Survival of mice. P > 0.05.
Figure 7. Propionate does not sensitize to mild infection by E. coli and S. pneumoniae.
BALB/c mice were treated with or without 200 mM propionate in drinking water (A,C) or 1 g/kg propionate given i.p. every other day (B) for 3 weeks and challenged with E. coli (4 × 104 CFU i.p.; n = 10 per group; (A,B) or S. pneumoniae (104 CFU i.p.; n = 9–10; C). (A) Bacterial counts in blood 24 h post-infection and survival of mice. P = 0.9 and 0.7. (B,C) Survival of mice. P = 0.6 and 0.4.
Figure 8. Propionate does not impair passive immunization and protection to secondary infection.
Anti-K. pneumoniae (A), anti-S. pneumoniae (B) and anti-C. albicans (C) IgG titers in BALB/c mice surviving infection with 20 CFU K. pneumoniae (n = 4 control and 5 propionate-treated mice; Fig. 5B), 104 CFU S. pneumoniae (n = 9 control and 10 propionate-treated mice; Fig. 7C) and 4 × 104 CFU C. albicans (n = 9 control and 9 propionate-treated mice, serum was collected 3 weeks post-infection). Box and min-to-max whisker plots represent the OD450 nm using plasma (diluted 1/200) collected on day 21 after infection. P = 0.1, 0.01 and 0.02, respectively. No signal was detected using plasma from uninfected mice. (D,E) BALB/c mice (n = 18–21 per group) were treated with or without 200 mM propionate in drinking water for 3 weeks, challenged i.n. with 80 CFU S. pneumoniae, and used for subsequent experimentation 3 weeks later. (D) Sera collected from 8 water and 11 propionate-treated mice were pooled and transferred (120 μl i.p.) into naive mice (n = 10 per group) infected 24 h later with 4 × 106 CFU S. pneumoniae (~100 x LD100). Survival was monitored for 21 days. P = 0.6. (E) Propionate treatment was withdrawn. Mice (n = 10 per group) were infected with 107 CFU S. pneumoniae (~250 x LD100). Survival was monitored for 21 days. P = 0.8.
Similar articles
- Short-chain fatty acids inhibit the activation of T lymphocytes and myeloid cells and induce innate immune tolerance.
Porbahaie M, Hummel A, Saouadogo H, Coelho RML, Savelkoul HFJ, Teodorowicz M, van Neerven RJJ. Porbahaie M, et al. Benef Microbes. 2023 Sep 1;14(4):401-419. doi: 10.1163/18762891-20220113. Benef Microbes. 2023. PMID: 38661366 - Sirtuin 5 Deficiency Does Not Compromise Innate Immune Responses to Bacterial Infections.
Heinonen T, Ciarlo E, Théroude C, Pelekanou A, Herderschee J, Le Roy D, Roger T. Heinonen T, et al. Front Immunol. 2018 Nov 20;9:2675. doi: 10.3389/fimmu.2018.02675. eCollection 2018. Front Immunol. 2018. PMID: 30515162 Free PMC article. - Innate immune recognition of microbial cell wall components and microbial strategies to evade such recognitions.
Sukhithasri V, Nisha N, Biswas L, Anil Kumar V, Biswas R. Sukhithasri V, et al. Microbiol Res. 2013 Aug 25;168(7):396-406. doi: 10.1016/j.micres.2013.02.005. Epub 2013 Apr 8. Microbiol Res. 2013. PMID: 23578963 Review. - Immunomodulatory roles of microbiota-derived short-chain fatty acids in bacterial infections.
Ranjbar R, Vahdati SN, Tavakoli S, Khodaie R, Behboudi H. Ranjbar R, et al. Biomed Pharmacother. 2021 Sep;141:111817. doi: 10.1016/j.biopha.2021.111817. Epub 2021 Jun 11. Biomed Pharmacother. 2021. PMID: 34126349 Review.
Cited by
- Origin, Differentiation, and Function of Intestinal Macrophages.
Bain CC, Schridde A. Bain CC, et al. Front Immunol. 2018 Nov 27;9:2733. doi: 10.3389/fimmu.2018.02733. eCollection 2018. Front Immunol. 2018. PMID: 30538701 Free PMC article. Review. - Review: The Nutritional Management of Multiple Sclerosis With Propionate.
Tobin D, Vige R, Calder PC. Tobin D, et al. Front Immunol. 2021 Jul 28;12:676016. doi: 10.3389/fimmu.2021.676016. eCollection 2021. Front Immunol. 2021. PMID: 34394076 Free PMC article. Review. - Staphylococcus aureus Lipase 3 (SAL3) is a surface-associated lipase that hydrolyzes short chain fatty acids.
Kumar NG, Contaifer D Jr, Wijesinghe DS, Jefferson KK. Kumar NG, et al. PLoS One. 2021 Oct 7;16(10):e0258106. doi: 10.1371/journal.pone.0258106. eCollection 2021. PLoS One. 2021. PMID: 34618844 Free PMC article. - Sirtuin 2 Deficiency Increases Bacterial Phagocytosis by Macrophages and Protects from Chronic Staphylococcal Infection.
Ciarlo E, Heinonen T, Théroude C, Herderschee J, Mombelli M, Lugrin J, Pfefferlé M, Tyrrell B, Lensch S, Acha-Orbea H, Le Roy D, Auwerx J, Roger T. Ciarlo E, et al. Front Immunol. 2017 Aug 28;8:1037. doi: 10.3389/fimmu.2017.01037. eCollection 2017. Front Immunol. 2017. PMID: 28894448 Free PMC article. - Host-mycobiome metabolic interactions in health and disease.
Begum N, Harzandi A, Lee S, Uhlen M, Moyes DL, Shoaie S. Begum N, et al. Gut Microbes. 2022 Jan-Dec;14(1):2121576. doi: 10.1080/19490976.2022.2121576. Gut Microbes. 2022. PMID: 36151873 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical