Intravenous Treatment with a Long-Chain Omega-3 Lipid Emulsion Provides Neuroprotection in a Murine Model of Ischemic Stroke - A Pilot Study - PubMed (original) (raw)
Intravenous Treatment with a Long-Chain Omega-3 Lipid Emulsion Provides Neuroprotection in a Murine Model of Ischemic Stroke - A Pilot Study
Dirk Berressem et al. PLoS One. 2016.
Abstract
Single long-chain omega-3 fatty acids (e.g. docosahexaenoic acid (DHA) or eicosapentaenoic acid (EPA)) are known for their neuroprotective properties associated with ischemic stroke. This pilot study aimed to test the effectiveness of an acute treatment with a long-chain omega-3 lipid emulsion (Omegaven 10%®, OGV) that contains fish oil (DHA 18 mg/ml; EPA 21 mg/ml) and α-tocopherol (0.2 mg/ml) in a transient middle cerebral artery occlusion (MCAO) model of ischemic stroke in mice. For this purpose, female CD-1 mice were anesthetized and subjected to 90 minutes of MCAO. To reflect a clinically relevant situation for an acute treatment, either after induction of stroke or after reperfusion, a single dose of OGV was injected intravenously into the tail vein (5 ml/kg b.w.). A neurological severity score was used to assess motor function and neurological outcome. Stroke-related parameters were determined 24 hours after MCAO. Microdialysis was used to collect samples from extracellular space of the striatum. Mitochondrial function was determined in isolated mitochondria or dissociated brain cells. Inflammation markers were measured in brain homogenate. According to control experiments, neuroprotective effects could be attributed to the long-chain omega-3 content of the emulsion. Intravenous injection of OGV reduced size and severity of stroke, restored mitochondrial function, and prevented excitotoxic glutamate release. Increases of pro-inflammatory markers (COX-2 and IL-6) were attenuated. Neurological severity scoring and neurochemical data demonstrated that acute OGV treatment shortly after induction of stroke was most efficient and able to improve short-term neurological outcome, reflecting the importance of an acute treatment to improve the outcome. Summarising, acute treatment of stroke with a single intravenous dose of OGV provided strong neuroprotective effects and was most effective when given immediately after onset of ischemia. As OGV is an approved fishoil emulsion for parenteral nutrition in humans, our results may provide first translational data for a possible early management of ischemic stroke with administration of OGV to prevent further brain damage.
Conflict of interest statement
This study was funded by Fresenius Kabi. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
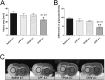
Fig 1. Effects of OGV and control emulsions when applied at reperfusion.
Saline, Lipofundin® (LPF) and d-α-tocopheryl polyethylene glycol 1000 succinate (TPGS) in doses equal to OGV when injected at reperfusion (a.r.). (A) Infarct areas and (B) differences in grayscale for LPF, TPGS, and Omegaven 10% (OGV) vs. Saline; n = 6. Mean ± SEM, p*<0.05; p**<0.01; p***<0.001; One-Way ANOVA with Tukey post-test. (C) Representative striatal brain slices for determination of differences in grayscale for each group. Density of grayscale in a representative area from core of infarction (solid circle) was subtracted from corresponding grayscale of contralateral control area (dashed circle).
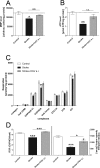
Fig 2. Marker of mitochondrial function after treatment at reperfusion.
Sham-operated mice. (Control) versus stroke control group that received saline (Stroke) and stroke treatment group that received OGV at reperfusion (a.r.). (A) Mitochondrial membrane potential (MMP)- and (B) Adenosine triphosphate (ATP)-levels as measured 24 hours after reperfusion in dissociated brain cells, n = 8; (C) Respiration [pmol oxygen/(s* mg protein)] of different complexes of the respiratory chain were determined in isolated mitochondria, n = 6; (D) Respiratory control ratio (RCR) that indicates the coupling of mitochondrial respiration chain and citrate synthase activity that represents a quantitative marker for mitochondrial mass, n = 6. Mean ± SEM, p*<0.05; p**<0.01; p***<0.001; One-Way ANOVA with Tukey post-test.
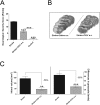
Fig 3. Effects of OGV when given at stroke (a.s.).
(A) Neurobehavioral assessment (refer to S1 Table) for sham-operated mice (Control) versus control group that received saline (Stroke) and treatment group that received OGV at stroke (a.s.), (B) Representative brain slices for determination of infarct volume and differences in grayscale. (C) Effect on infarct volume and grayscale levels, n = 8. Mean ± SEM, p*<0.05; p**<0.01; p***<0.001; (A) One-Way ANOVA with Tukey post-test, (C) t-test.
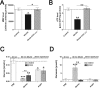
Fig 4. Marker of mitochondrial function after treatment at stroke.
Sham mice (Control) versus stroke control group that received saline (Stroke) and stroke treatment group that received OGV at stroke (a.s.). (A) Mitochondrial membrane potential (MMP)- and (B) Adenosine triphosphate (ATP)-levels as measured in dissociated brain cells 24 hours after reperfusion. Energy metabolite levels in striatal core region of stroke for (C) glucose and (D) glutamate as determined by microdialysis 30 minutes before (PRE), 90 minutes during (Stroke) and 30 minutes after (POST) stroke surgery, n = 8. Mean ± SEM, p*<0.05; p**<0.01;p***<0.001; One-Way ANOVA with Tukey post-test.
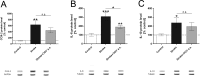
Fig 5. Western Blot analysis of brain homogenates.
Sham-operated mice (Control) versus control group that received saline (Stroke) and treatment group that received OGV at stroke (a.s.). (A) COX-2-, (B) IL-6-, and (C) IL-10 protein levels, n = 8. Mean ± SEM, p*<0.05; p**<0.01; p***<0.001; One-Way ANOVA with Tukey post-test.
Similar articles
- Plasma and erythrocyte uptake of omega-3 fatty acids from an intravenous fish oil based lipid emulsion in patients with advanced oesophagogastric cancer.
Eltweri AM, Thomas AL, Fisk HL, Arshad A, Calder PC, Dennison AR, Bowrey DJ. Eltweri AM, et al. Clin Nutr. 2017 Jun;36(3):768-774. doi: 10.1016/j.clnu.2016.06.001. Epub 2016 Jun 7. Clin Nutr. 2017. PMID: 27342748 - Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window.
Belayev L, Liu Y, Zhao W, Busto R, Ginsberg MD. Belayev L, et al. Stroke. 2001 Feb;32(2):553-60. doi: 10.1161/01.str.32.2.553. Stroke. 2001. PMID: 11157196 - N-3 fatty acid rich triglyceride emulsions are neuroprotective after cerebral hypoxic-ischemic injury in neonatal mice.
Williams JJ, Mayurasakorn K, Vannucci SJ, Mastropietro C, Bazan NG, Ten VS, Deckelbaum RJ. Williams JJ, et al. PLoS One. 2013;8(2):e56233. doi: 10.1371/journal.pone.0056233. Epub 2013 Feb 20. PLoS One. 2013. PMID: 23437099 Free PMC article. - Fish oil lipid emulsions and immune response: what clinicians need to know.
Waitzberg DL, Torrinhas RS. Waitzberg DL, et al. Nutr Clin Pract. 2009 Aug-Sep;24(4):487-99. doi: 10.1177/0884533609339071. Nutr Clin Pract. 2009. PMID: 19605803 Review. - Impact of Providing a Combination Lipid Emulsion Compared With a Standard Soybean Oil Lipid Emulsion in Children Receiving Parenteral Nutrition: A Systematic Review and Meta-Analysis.
Finn KL, Chung M, Rothpletz-Puglia P, Byham-Gray L. Finn KL, et al. JPEN J Parenter Enteral Nutr. 2015 Aug;39(6):656-67. doi: 10.1177/0148607114542515. Epub 2014 Jul 23. JPEN J Parenter Enteral Nutr. 2015. PMID: 25057053 Review.
Cited by
- The mechanism of microglia-mediated immune inflammation in ischemic stroke and the role of natural botanical components in regulating microglia: A review.
Zeng J, Bao T, Yang K, Zhu X, Wang S, Xiang W, Ge A, Zeng L, Ge J. Zeng J, et al. Front Immunol. 2023 Feb 2;13:1047550. doi: 10.3389/fimmu.2022.1047550. eCollection 2022. Front Immunol. 2023. PMID: 36818470 Free PMC article. Review. - Modulation of secretory factors by lipofundin contributes to its anti‑neuroinflammatory effects.
Chen MS, Hu CL, Jiang SK, Chong ZY, Chen JC. Chen MS, et al. Exp Ther Med. 2024 Feb 27;27(4):169. doi: 10.3892/etm.2024.12456. eCollection 2024 Apr. Exp Ther Med. 2024. PMID: 38476917 Free PMC article. - The Effect of Mitochondrial Supplements on Mitochondrial Activity in Children with Autism Spectrum Disorder.
Delhey LM, Nur Kilinc E, Yin L, Slattery JC, Tippett ML, Rose S, Bennuri SC, Kahler SG, Damle S, Legido A, Goldenthal MJ, Frye RE. Delhey LM, et al. J Clin Med. 2017 Feb 13;6(2):18. doi: 10.3390/jcm6020018. J Clin Med. 2017. PMID: 28208802 Free PMC article. - Measuring Respiration in Isolated Murine Brain Mitochondria: Implications for Mechanistic Stroke Studies.
Sperling JA, Sakamuri SSVP, Albuck AL, Sure VN, Evans WR, Peterson NR, Rutkai I, Mostany R, Satou R, Katakam PVG. Sperling JA, et al. Neuromolecular Med. 2019 Dec;21(4):493-504. doi: 10.1007/s12017-019-08552-8. Epub 2019 Jun 6. Neuromolecular Med. 2019. PMID: 31172441 Free PMC article. - Does Alpha-lipoic Acid Supplementation Modulate Cardiovascular Risk Factors in Patients with Stroke? A Randomized, Double-blind Clinical Trial.
Mohammadi V, Khorvash F, Feizi A, Askari G. Mohammadi V, et al. Int J Prev Med. 2018 Apr 5;9:34. doi: 10.4103/ijpvm.IJPVM_32_17. eCollection 2018. Int J Prev Med. 2018. PMID: 29721235 Free PMC article.
References
- Jauch EC, Saver JL, Adams HP, Bruno A, Connors JJB, Demaerschalk BM, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870–947. 10.1161/STR.0b013e318284056a - DOI - PubMed
MeSH terms
Substances
Grants and funding
This study was partly supported by Fresenius Kabi Deutschland GmbH, Bad Homburg, Deutschland. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials