Red blood cell transfusion and skeletal muscle tissue oxygenation in anaemic haematologic outpatients - PubMed (original) (raw)
Red blood cell transfusion and skeletal muscle tissue oxygenation in anaemic haematologic outpatients
Matej Podbregar et al. Radiol Oncol. 2016.
Abstract
Background: Stored red blood cells (RBCs) accumulate biochemical and biophysical changes, known as storage lesion. The aim of this study was to re-challenge current data that anaemia in chronically anaemic haematology patients is not associated with low skeletal muscle tissue oxygen (StO2), and that RBC storage age does not influence the tissue response after ischaemic provocation, using near-infrared spectroscopy.
Patients and methods: Twenty-four chronic anaemic haematology patients were included. Thenar skeletal muscle StO2 was measured at rest (basal StO2), with vascular occlusion testing (upslope StO2, maximum StO2) before and after transfusion.
Results: Basal StO2 was low (53% ± 7%). Average RBC storage time was 10.5 ± 3.9 days. Effects of RBC transfusions were as follows: basal StO2 and upslope StO2 did not change significantly; maximum StO2 increased compared to baseline (64 ± 14% vs. 59 ± 10%, p = 0.049). Change of basal StO2, upslope StO2 and maximum StO2 was negatively related to age of RBCs. The decrease of maximum StO2 was predicted (sensitivity 70%, specificity 100%), after receiving RBCs ≥ 10days old.
Discussion: Resting skeletal muscle StO2 in chronic anaemic patients is low. RBC storage time affects skeletal muscle StO2 in the resting period and after ischaemic provocation.
Keywords: red blood cells; skeletal muscle; storage lesion; tissue oxygenation; transfusion.
Figures
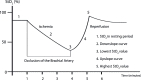
Figure 1
Schematic presentation of thenar skeletal muscle StO2 before, during and after the vascular occlusion tests. Before the vascular occlusion, the StO2 is measured in the resting period (1, basal StO2). During the vascular occlusion, the StO2 gradually decreases. The rate of this decrease is determined from the curve as the downslope StO2 (2; %/min), as a surrogate of the tissue oxygen consumption. After reaching the predetermined minimum StO2, present here as 40% StO2 (3), the vascular occlusion is released, and the StO2 begins to rise again. The rate of this increase is determined from the curve as the upslope StO2 (4; %/min), as a surrogate marker of the microcirculatory reactivity. After the release of the occlusion, the StO2 increases to higher values compared to the basal StO2 due to post-ischaemic vasodilatation (5, maximum StO2). The StO2 then slowly returns to the basal StO2.
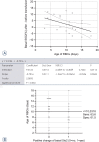
Figure 2
Effects of the age of the RBCs for the transfusions on the basal StO2. (A) Regression/analysis of variance. (B) Roc analysis, interactive dot diagram for optimal effect separation. Prediction line (solid lines); 95% confidence line (dashed lines)
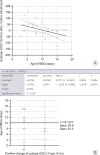
Figure 3
Effects of the age of the RBCs for the transfusions on the upslope StO2. (A) Regression/analysis of variance. (B) ROC analysis, interactive dot diagram for optimal effect separation. Prediction line (solid lines); 95% confidence line (dashed lines
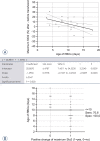
Figure 4
Effects of the age of the RBCs for the transfusions on the maximum StO2. (A) Regression/analysis of variance. (B) ROC analysis, interactive dot diagram for optimal effect separation Prediction line (solid lines); 95% confidence line (dashed lines)
Similar articles
- Near-infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation.
Creteur J, Neves AP, Vincent JL. Creteur J, et al. Crit Care. 2009;13 Suppl 5(Suppl 5):S11. doi: 10.1186/cc8009. Epub 2009 Nov 30. Crit Care. 2009. PMID: 19951383 Free PMC article. - Microcirculatory effects of the transfusion of leukodepleted or non-leukodepleted red blood cells in patients with sepsis: a pilot study.
Donati A, Damiani E, Luchetti M, Domizi R, Scorcella C, Carsetti A, Gabbanelli V, Carletti P, Bencivenga R, Vink H, Adrario E, Piagnerelli M, Gabrielli A, Pelaia P, Ince C. Donati A, et al. Crit Care. 2014 Feb 17;18(1):R33. doi: 10.1186/cc13730. Crit Care. 2014. PMID: 24528648 Free PMC article. Clinical Trial. - The effects of cryopreserved red blood cell transfusion on tissue oxygenation in obese trauma patients.
McCully BH, Underwood SJ, Kiraly L, Holcomb JB, Robinson BRH, Minei JP, Stewart RM, Cotton BA, Gordon NT, Martin DT, Rick EA, Dean RK, Wiles C, Anderson N, Schreiber MA. McCully BH, et al. J Trauma Acute Care Surg. 2018 Jan;84(1):104-111. doi: 10.1097/TA.0000000000001717. J Trauma Acute Care Surg. 2018. PMID: 29267183 - Association between static and dynamic thenar near-infrared spectroscopy and mortality in patients with sepsis: a systematic review and meta-analysis.
Neto AS, Pereira VG, Manetta JA, Espósito DC, Schultz MJ. Neto AS, et al. J Trauma Acute Care Surg. 2014 Jan;76(1):226-33. doi: 10.1097/TA.0b013e3182a9221f. J Trauma Acute Care Surg. 2014. PMID: 24368385 Review. - Red blood cell storage lesion.
Obrador R, Musulin S, Hansen B. Obrador R, et al. J Vet Emerg Crit Care (San Antonio). 2015 Mar-Apr;25(2):187-99. doi: 10.1111/vec.12252. Epub 2014 Nov 26. J Vet Emerg Crit Care (San Antonio). 2015. PMID: 25428860 Review.
Cited by
- Transfusion Decision Making in Pediatric Critical Illness.
Markham C, Small S, Hovmand P, Doctor A. Markham C, et al. Pediatr Clin North Am. 2017 Oct;64(5):991-1015. doi: 10.1016/j.pcl.2017.06.003. Pediatr Clin North Am. 2017. PMID: 28941545 Free PMC article. Review. - Association between sarcopenia and hemoglobin level: a systematic review and meta-analysis.
Wang H, Lin P. Wang H, et al. Front Med (Lausanne). 2024 Jul 25;11:1424227. doi: 10.3389/fmed.2024.1424227. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39118670 Free PMC article. - Measurement of Tissue Oxygen as a Novel Approach to Optimizing Red Blood Cell Quality Assessment.
Buehler PW, Flood AB, Swartz HM. Buehler PW, et al. Adv Exp Med Biol. 2021;1269:379-386. doi: 10.1007/978-3-030-48238-1_60. Adv Exp Med Biol. 2021. PMID: 33966246 - Consensus of the Brazilian association of hematology, hemotherapy and cellular therapy on patient blood management: Anemia tolerance mechanisms.
Rodrigues RDR, Brunetta DM, Costa L, Benites BD, Magnus MM, Alves SOC, De Santis GC, Rizzo SRCP, Rabello G, Junior DML. Rodrigues RDR, et al. Hematol Transfus Cell Ther. 2024 Apr;46 Suppl 1(Suppl 1):S77-S82. doi: 10.1016/j.htct.2024.02.010. Epub 2024 Mar 19. Hematol Transfus Cell Ther. 2024. PMID: 38575401 Free PMC article. - Electron paramagnetic resonance oximetry as a novel approach to monitor the effectiveness and quality of red blood cell transfusions.
Hou H, Baek JH, Zhang H, Wood F, Gao Y, Flood AB, Swartz HM, Buehler PW. Hou H, et al. Blood Transfus. 2019 Jul;17(4):296-306. doi: 10.2450/2019.0037-19. Epub 2019 May 16. Blood Transfus. 2019. PMID: 31184583 Free PMC article.
References
- Adamson WJ, Longo LD. Longo LD, Fauci SA, Kasper LD, Harrison’s principles of internal medicine. 18th edition. New York: McGrawHill Medical; 2012. Anemia and polycythemia; pp. 448–57.
- Madjdpour C, Spahn DR.. Allogeneic red blood cell transfusions: efficacy, risks, alternatives and indications. Br J Anaesth. 2005;95:33–42. - PubMed
- Klein HG, Spahn DR, Carson JL.. Red blood cell transfusion in clinical practice. Lancet. 2007;370:415–26. - PubMed
- Ferrari M, Giannini I, Sideri G, Zanette E.. Continuous non invasive monitoring of human brain by near infrared spectroscopy. Adv Exp Med Biol. 1985;191:873–82. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources