Inflammatory processes in cardiovascular disease: a route to targeted therapies - PubMed (original) (raw)
Review
Inflammatory processes in cardiovascular disease: a route to targeted therapies
Neil Ruparelia et al. Nat Rev Cardiol. 2017 Mar.
Erratum in
- Inflammatory processes in cardiovascular disease: a route to targeted therapies.
Ruparelia N, Chai JT, Fisher EA, Choudhury RP. Ruparelia N, et al. Nat Rev Cardiol. 2017 May;14(5):314. doi: 10.1038/nrcardio.2017.33. Epub 2017 Mar 16. Nat Rev Cardiol. 2017. PMID: 28300082 No abstract available.
Abstract
Inflammatory processes are firmly established as central to the development and complications of cardiovascular diseases. Elevated levels of inflammatory markers have been shown to be predictive of future cardiovascular events. The specific targeting of these processes in experimental models has been shown to attenuate myocardial and arterial injury, reduce disease progression, and promote healing. However, the translation of these observations and the demonstration of clear efficacy in clinical practice have been disappointing. A major limitation might be that tools currently used to measure 'inflammation' are insufficiently precise and do not provide information about disease site and activity, or discriminate between functionally important activation pathways. The challenge, therefore, is to make measures of inflammation that are more meaningful, and which can guide specific targeted therapies. In this Review, we consider the roles of inflammatory processes in the related pathologies of atherosclerosis and acute myocardial infarction, by providing an evaluation of the known and emerging inflammatory pathways. We highlight contemporary techniques to characterize and quantify inflammation, and consider how they might be used to guide specific treatments. Finally, we discuss emerging opportunities in the field, including their current limitations and challenges that are the focus of ongoing study.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures
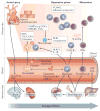
Figure 1
Biological pathways central to the pathogenesis of acute myocardial infarction (AMI). Immediately following AMI, a number of local processes are activated with release of reactive oxygen species and cytokines with infiltration of circulating neutrophils and monocytes resulting in acute myocardial injury. Simultaneously a number of remote sites are also activated (e.g. spleen, bone marrow) via signalling pathways that result in further activation of the immune system and injury. Following this, a reparative phase ensues predominantly mediated by monocytes and T-lymphocytes resulting in tissue repair and recovery with upregulation of processes involved in angiogenesis and extracellular matrix deposition. Abbreviations: ROS: reactive oxygen species; TLR: toll-like receptors; DAMPS: damage associated molecular patterns; HSP: heat shock proteins; HMGB1: high mobility group box 1 protein; VCAM: vascular cell adhesion molecule; NLR: NOD-like receptor; NLRP3: NOD-like receptor family pyrin domain containing 3; IL: interleukin; TNF: tumour necrosis factor; IFN: interferon; CX3CR1: CX3 chemokine receptor 1; miRNA: micro ribonucleic acid; CRP: C reactive protein; SAA: serum amyloid A.
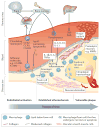
Figure 2
Biological processes central to the pathogenesis of atherosclerosis. Endothelial cells, lymphocytes, smooth muscle cells, monocytes, and macrophages are all involved in the pathogenesis of atherosclerosis from earliest foam cell formation through to development of advanced plaques. Initial activation of the endothelium from disruptions to normal shear stress result and facilitate deposition of lipid in the subendothelial space. Endothelial activation also promotes recruitment of circulating monocytes where they terminally differentiate into macrophage or differentiate and locally proliferate into distinct functional phenotypes. Activated macrophages take up lipid and results in their transformation into macrophage-derived foam cells. As the foam cell population grows within lesions in the arterial wall, the rate of accumulation exceeds the rate of clearance, and eventually the foam cells coalesce into a lipid-rich necrotic core. Abbreviations: VCAM: vascular cell adhesion molecule; ICAM: intercellular cell adhesion molecule; CCR: chemokine-chemokine receptor; CX3CR1: CX3 chemokine receptor; IL: interleukin; TNF: tumour necrosis factor; ApoB: Apoliporotein B; CRP: C reactive protein; SAA: serum amyloid A; MMP: matrix metalloproteinase; NLRP3: NOD-like receptor family pyrin domain containing 3; VSMC: vascular smooth muscle cells.
Comment in
- Inflammation: DNases prevent clots formed by neutrophil extracellular traps.
Lim GB. Lim GB. Nat Rev Cardiol. 2018 Feb;15(2):69. doi: 10.1038/nrcardio.2017.216. Epub 2017 Dec 21. Nat Rev Cardiol. 2018. PMID: 29263449 No abstract available.
Similar articles
- Anti-inflammatory drugs as promising cardiovascular treatments.
Huet F, Akodad M, Fauconnier J, Lacampagne A, Roubille F. Huet F, et al. Expert Rev Cardiovasc Ther. 2017 Feb;15(2):109-125. doi: 10.1080/14779072.2017.1273771. Epub 2016 Dec 28. Expert Rev Cardiovasc Ther. 2017. PMID: 28010154 Review. - Targeting the adaptive immune system: new strategies in the treatment of atherosclerosis.
Zarzycka B, Nicolaes GA, Lutgens E. Zarzycka B, et al. Expert Rev Clin Pharmacol. 2015 May;8(3):297-313. doi: 10.1586/17512433.2015.1025052. Epub 2015 Apr 4. Expert Rev Clin Pharmacol. 2015. PMID: 25843158 Review. - Critical roles of inflammation in atherosclerosis.
Moriya J. Moriya J. J Cardiol. 2019 Jan;73(1):22-27. doi: 10.1016/j.jjcc.2018.05.010. Epub 2018 Jun 12. J Cardiol. 2019. PMID: 29907363 Review. - Novel anti-inflammatory therapies for the treatment of atherosclerosis.
Khan R, Spagnoli V, Tardif JC, L'Allier PL. Khan R, et al. Atherosclerosis. 2015 Jun;240(2):497-509. doi: 10.1016/j.atherosclerosis.2015.04.783. Epub 2015 Apr 18. Atherosclerosis. 2015. PMID: 25917947 Review. - Preclinical mouse models and methods for the discovery of the causes and treatments of atherosclerosis.
Hewing B, Fisher EA. Hewing B, et al. Expert Opin Drug Discov. 2012 Mar;7(3):207-16. doi: 10.1517/17460441.2012.660143. Epub 2012 Feb 10. Expert Opin Drug Discov. 2012. PMID: 22468952 Free PMC article. Review.
Cited by
- Implications of endoplasmic reticulum stress and autophagy in aging and cardiovascular diseases.
Ma C, Liu Y, Fu Z. Ma C, et al. Front Pharmacol. 2024 Jul 25;15:1413853. doi: 10.3389/fphar.2024.1413853. eCollection 2024. Front Pharmacol. 2024. PMID: 39119608 Free PMC article. Review. - The interplay of galectins-1, -3, and -9 in the immune-inflammatory response underlying cardiovascular and metabolic disease.
Mansour AA, Krautter F, Zhi Z, Iqbal AJ, Recio C. Mansour AA, et al. Cardiovasc Diabetol. 2022 Nov 19;21(1):253. doi: 10.1186/s12933-022-01690-7. Cardiovasc Diabetol. 2022. PMID: 36403025 Free PMC article. Review. - Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles.
Bonora M, Wieckowski MR, Sinclair DA, Kroemer G, Pinton P, Galluzzi L. Bonora M, et al. Nat Rev Cardiol. 2019 Jan;16(1):33-55. doi: 10.1038/s41569-018-0074-0. Nat Rev Cardiol. 2019. PMID: 30177752 Free PMC article. Review. - Damage-Associated Molecular Patterns in Inflammatory Diseases.
Roh JS, Sohn DH. Roh JS, et al. Immune Netw. 2018 Aug 13;18(4):e27. doi: 10.4110/in.2018.18.e27. eCollection 2018 Aug. Immune Netw. 2018. PMID: 30181915 Free PMC article. Review. - Cardiovascular Inflammaging: Mechanisms and Translational Aspects.
Barcena ML, Aslam M, Pozdniakova S, Norman K, Ladilov Y. Barcena ML, et al. Cells. 2022 Mar 16;11(6):1010. doi: 10.3390/cells11061010. Cells. 2022. PMID: 35326461 Free PMC article. Review.
References
- Libby P. Inflammation and cardiovascular disease mechanisms. The American journal of clinical nutrition. 2006;83:456S–460S. - PubMed
- Biasucci LM, et al. Increasing levels of interleukin (IL)-1Ra and IL-6 during the first 2 days of hospitalization in unstable angina are associated with increased risk of in-hospital coronary events. Circulation. 1999;99:2079–2084. - PubMed
- Valgimigli M, et al. Tumor necrosis factor-alpha receptor 1 is a major predictor of mortality and new-onset heart failure in patients with acute myocardial infarction: the Cytokine-Activation and Long-Term Prognosis in Myocardial Infarction (C-ALPHA) study. Circulation. 2005;111:863–870. doi: 10.1161/01.CIR.0000155614.35441.69. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MR/K00266X/1/MRC_/Medical Research Council/United Kingdom
- R01 HL129433/HL/NHLBI NIH HHS/United States
- PG/12/46/29673/BHF_/British Heart Foundation/United Kingdom
- P01 HL092969/HL/NHLBI NIH HHS/United States
- R01 HL084312/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical