In vitro patterning of pluripotent stem cell-derived intestine recapitulates in vivo human development - PubMed (original) (raw)
. 2017 Mar 15;144(6):1045-1055.
doi: 10.1242/dev.138453. Epub 2016 Dec 7.
Affiliations
- PMID: 27927684
- PMCID: PMC5358103
- DOI: 10.1242/dev.138453
In vitro patterning of pluripotent stem cell-derived intestine recapitulates in vivo human development
Yu-Hwai Tsai et al. Development. 2017.
Abstract
The intestine plays a central role in digestion, nutrient absorption and metabolism, with individual regions of the intestine having distinct functional roles. Many examples of region-specific gene expression in the adult intestine are known, but how intestinal regional identity is established during development is a largely unresolved issue. Here, we have identified several genes that are expressed in a region-specific manner in the developing human intestine. Using human embryonic stem cell-derived intestinal organoids, we demonstrate that the duration of exposure to active FGF and WNT signaling controls regional identity. Short-term exposure to FGF4 and CHIR99021 (a GSK3β inhibitor that stabilizes β-catenin) resulted in organoids with gene expression patterns similar to developing human duodenum, whereas longer exposure resulted in organoids similar to ileum. When region-specific organoids were transplanted into immunocompromised mice, duodenum-like organoids and ileum-like organoids retained their regional identity, demonstrating that regional identity of organoids is stable after initial patterning occurs. This work provides insights into the mechanisms that control regional specification of the developing human intestine and provides new tools for basic and translational research.
Keywords: Human; Intestine; Organoid; Patterning; Pluripotent stem cells.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests
The authors declare no competing or financial interests.
Figures
Fig. 1.
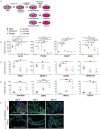
Identification of regionally expressed molecular markers in the human fetal intestine. (A) Genes known to be enriched in the proximal developing mouse intestine, including PDX1, GATA4, TM4SF4 and ONECUT2 were examined in different regions of the human fetal intestine (_n_=5 individual biological specimens; proximal, blue; middle, red; distal, green). (B) Genes know to be enriched in the distal developing mouse intestine, including GUCA2A, OSR2, MUC2 and FZD10 were examined in different regions of the human fetal intestine (_n_=5 individual biological specimens; proximal, blue; middle, red; distal, green). (C) Enrichment of PDX1 and GATA4 protein as assessed by immunofluorescence and of ONECUT2 mRNA as assessed by in situ hybridization was confirmed in the proximal region of the fetal intestine, whereas GUCA2A mRNA and MUC2 protein were enriched in the distal fetal intestine when assessed by in situ hybridization and immunofluorescence, respectively. Scale bars: 200 μm.
Fig. 2.
Human intestinal organoids are patterned by FGF and WNT signaling. (A) Schematic of experimental design showing spheroids generated in culture over increasing periods of time. (B) Expression of OCT4, FOXA2, SOX17 and CDX2 during differentiation in undifferentiated hESCs, in endoderm and hindgut (4 days after FGF4/CHIR99021), and in organoids derived from d5, d7 and d10 cultures (d5, blue; d7, red; d10, green). (C) Markers shown to be enriched in the human fetal duodenum (Fig. 1), including PDX1, GATA4, TM4SF4 and ONECUT2 were examined in d5, d7 and d10 organoids. (D) Markers shown to be enriched in the human fetal ileum (Fig. 1), including MUC2, OSR2, MUC2 and FZD10 were examined in d5, d7 and d10 organoids. (E) Immunofluorescence demonstrated that PDX1 protein expression was enriched in d5 organoids, whereas MUC2 protein expression was enriched in d7 and d10 organoids. Scale bars: 200 μm.
Fig. 3.
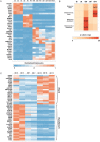
Bioinformatic identification of stage-enriched genes and comparison with published datasets. (A) Non-negative matrix factorization was used to identify stage-enriched genes. A normalized and curated heatmap shows representative genes (the full list is in
Table S1
). ES, human embryonic stem cells; DE, definitive endoderm; OD5, day 5 organoids; OD7, day 7 organoids; OD10, day 10 organoids. (B) Enriched genes in d5, d7 and d10 organoids were compared with published lists of genes whose expression is regionally restricted to the duodenum or the ileum. A hypergeometric test was used to determine the level of significance of overlapping gene sets. (C) Heatmap of representative genes found to overlap in patterned organoids and in published datasets shows enrichment for ileal genes in d10 organoids and for duodenal genes in d5 organoids.
Fig. 4.
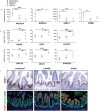
Validation of region-enriched genes in human fetal tissue. (A) qRT-PCR showing genes enriched in the human fetal proximal small intestine (_n_=5 individual biological specimens; proximal, blue; middle, red; distal, green). (B) qRT-PCR showing genes enriched in the human fetal distal small intestine. (C) In situ hybridization of DMBT1 showing stronger expression in the proximal small intestine, and immunohistochemistry of FABP6 showing more abundant protein staining in the middle/distal regions of the human small intestine. Scale bars: 200 μm.
Fig. 5.
Organoids retain regionalization after maturation in vivo. (A) d5, d7, d10 organoids were harvested after maturation in vivo. Hematoxylin and Eosin staining reveals that transplanted tissue possesses villus- and crypt-like domains. (B) PDX1 is most highly enriched in d5 organoids. (C) GATA4 is most highly enriched in d5 organoids. (D) SATB2 is most highly enriched in d10 organoids. (E) FABP6 is enriched in both d7 and d10 organoids. Scale bars: 200 μm.
References
- Beck C. W. and Slack J. M. (1999). A developmental pathway controlling outgrowth of the Xenopus tail bud. Development 126, 1611-1620. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 DK007762/DK/NIDDK NIH HHS/United States
- U19 AI116482/AI/NIAID NIH HHS/United States
- U24 DK085532/DK/NIDDK NIH HHS/United States
- T32 HD007505/HD/NICHD NIH HHS/United States
- U01 DK103141/DK/NIDDK NIH HHS/United States
- P30 DK034933/DK/NIDDK NIH HHS/United States
- U01 DK085532/DK/NIDDK NIH HHS/United States
- R24 HD000836/HD/NICHD NIH HHS/United States
- DP5 OD012194/OD/NIH HHS/United States
- P50 GM081879/GM/NIGMS NIH HHS/United States
- U01 DK103147/DK/NIDDK NIH HHS/United States
- T32 DK094775/DK/NIDDK NIH HHS/United States
- K01 DK091415/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources