Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics - PubMed (original) (raw)
Electrotonic coupling of excitable and nonexcitable cells in the heart revealed by optogenetics
T Alexander Quinn et al. Proc Natl Acad Sci U S A. 2016.
Abstract
Electrophysiological studies of excitable organs usually focus on action potential (AP)-generating cells, whereas nonexcitable cells are generally considered as barriers to electrical conduction. Whether nonexcitable cells may modulate excitable cell function or even contribute to AP conduction via direct electrotonic coupling to AP-generating cells is unresolved in the heart: such coupling is present in vitro, but conclusive evidence in situ is lacking. We used genetically encoded voltage-sensitive fluorescent protein 2.3 (VSFP2.3) to monitor transmembrane potential in either myocytes or nonmyocytes of murine hearts. We confirm that VSFP2.3 allows measurement of cell type-specific electrical activity. We show that VSFP2.3, expressed solely in nonmyocytes, can report cardiomyocyte AP-like signals at the border of healed cryoinjuries. Using EM-based tomographic reconstruction, we further discovered tunneling nanotube connections between myocytes and nonmyocytes in cardiac scar border tissue. Our results provide direct electrophysiological evidence of heterocellular electrotonic coupling in native myocardium and identify tunneling nanotubes as a possible substrate for electrical cell coupling that may be in addition to previously discovered connexins at sites of myocyte-nonmyocyte contact in the heart. These findings call for reevaluation of cardiac nonmyocyte roles in electrical connectivity of the heterocellular heart.
Keywords: cardiac; electrophysiology; fibroblast; genetically-encoded voltage indicator; heterocellular coupling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
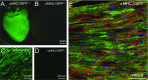
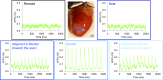
Fig. 1.
VSFP2.3 is highly expressed in myocytes of αMHC;VSFP2.3+;+ mice. (A_–_D) YFP fluorescence in (A and B) whole hearts and (C and D) histological sections of LV tissue immunolabeled with anti-YFP antibody showing (A) high and uniform VSFP2.3 expression localized at (C) the myocyte sarcolemma in αMHC;VSFP2.3+;+ mice, with (B and D) no discernible expression in αMHC;VSFP2.3+;− mice. (E) Representative immunohistochemical staining of VSFP2.3 (anti-YFP antibody; green), cell nuclei (DAPI; blue), and myocytes (antisarcomeric α-actinin antibody; red) in a histological section of LV tissue from an αMHC;VSFP2.3+;+ mouse, with extensive colabeling showing high specificity of myocyte targeting.
Fig. 2.
VSFP2.3 reports electrical activity of myocytes in αMHC;VSFP2.3+;+ hearts. (A) CFP (blue) and YFP (green) fluorescence (F; expressed as percentage change ΔF/Fo) simultaneously collected from the LV epicardium of an αMHC;VSFP2.3+;+ mouse heart showing electrical activity of myocytes, with signal enhancement by YFP/CFP ratiometry (black; expressed as percentage ΔR/Ro). (B) Simultaneously collected YFP (green) (Fig. S1_A_ and Movie S1) and voltage-sensitive fluorescent dye di-4-ANBDQPQ (red) (Fig. S1_B_ and Movie S2) fluorescence in αMHC;VSFP2.3+;+ mouse heart and their normalized comparison (Fn), illustrating the known slower de- and repolarization kinetics of VSFP2.3 compared to di-4-ANBDQPQ signals but otherwise, good AP detection fidelity.
Fig. S1.
Selected frames of (A) Movie S1 and (B) Movie S2 showing simultaneously collected (A) VSFP2.3 YFP and (B) voltage-sensitive fluorescent dye di-4-ANBDQPQ signals collected in an αMHC;VSFP2.3+;+ mouse heart. Comparison shows the difference in kinetics between the two signals. (Scale bar: 100 μm.)
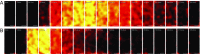
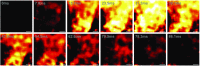
Fig. 3.
VSFP2.3 is highly expressed in nonmyocytes but not myocytes of WT1;VSFP2.3+;+ mice. (A_–_G) YFP fluorescence in histological sections of LV tissue showing increased VSFP2.3 expression as detected by anti-YFP antibody in interstitial nonmyocytes of (A) cryoinjured vs. (C) sham-operated WT1;VSFP2.3+;+ mice, lack of expression in WT1;VSFP2.3+;− mice (B) with and (D) without cryoinjury, (E) increase in expression from remote tissue to the scar border and into the scar in injured hearts, and (F and G) evenly distributed expression across the ventricular wall in sham hearts. Endo, subendocardium; Epi, subepicardium; Mid, mid-myocardium; NS, not significant. *P < 0.05; **P < 0.001. (H) Trichrome-stained longitudinal section of a cryoinjured heart showing the extent of scar tissue (collagen is stained bluish green, and myocytes are stained pink). RV, right ventricle. (I_–_N) Immunohistochemical staining of VSFP2.3 (green), cell nuclei (blue), and (I and J) myocytes (red) or (K_–_N) nonmyocytes (red) in histological sections of LV tissue from WT1;VSFP2.3+;+ mice, with colabeling showing lack of VSFP2.3 in myocytes of (I) cryoinjured and (J) sham-operated animals (a rare example of a VSFP2.3-expressing myocyte is shown in Inset) but high nonmyocyte-specific expression in the remote tissue of (K) cryoinjured and (L) sham hearts, which increases (M) in the scar border and (N) into the scar. Data are presented as mean ± SEM; n ≥ 26 per group.
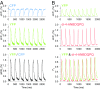
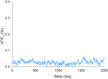
Fig. 4.
VSFP2.3 reports electrical activity of nonmyocytes at the scar border in WT1;VSFP2.3+;+ hearts. YFP fluorescence (F; expressed as percentage change ΔF/Fo) collected from remote, central scar, scar border, and adjacent tissue (both toward and away from the scar center) in a WT1;VSFP2.3+;+ mouse heart showing AP-like electrical activity in nonmyocytes at the scar border (indicating electrotonic signal transmission from myocytes to nonmyocytes) (Fig. S2 and Movie S3) and with lower amplitude in adjacent tissue (suggesting reduced passively conducted signal amplitude toward the scar center and reduced heterocellular electrotonic coupling away from the scar). No rhythmic polarizations were seen in central scar or remote tissue. [The number of fluorescence oscillations in border vs. adjacent tissue differed due to nonsimultaneous recordings.]
Fig. S2.
Selected frames of Movie S3 showing VSFP2.3 YFP signal collected at the scar border in a WT1;VSFP2.3+;+ mouse heart. The patchy, locally heterogeneous signals suggest a lack of contribution from VSFP2.3-expressing myocytes. (Scale bar: 100 μm.)
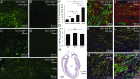
Fig. 5.
Tunneling nanotubes connecting nonmyocytes to myocytes at the scar border. (A) Thick section (275-nm) transmission electron micrograph showing extensive finger-like membrane protrusions (examples indicated by white arrowheads) extending from a nonmyocyte to a myocyte in the scar border zone. (B and C) Serial ET slices (separated by 20 nm in the z axis) reconstructed in the plane of membrane protrusions indicated by the (B) blue and (C) red boxes in A, including (C, row 5) a segmentation and 3D rendering representing nonmyocyte sarcolemma (green), myocyte surface sarcolemma (blue), and myocyte basement membrane (yellow). In addition to nonmyocyte-derived nanotubes that either (B) extend toward the myocyte basement membrane or (C) penetrate that to contact the myocyte sarcolemma, (D) occasionally both cell types possess sarcolemmal membrane extensions toward one other (Movie S4). M, myocyte; nonM, nonmyocyte.
Fig. S3.
CFP fluorescence collected from scar border tissue in a WT1;VSFP2.3+;+ mouse heart at the same location as the YFP signal shown in Fig. 4. No decrease in fluorescence indicating AP-like activity was detected because of the weak CFP signal, but also, no increase in fluorescence similar to that of YFP that would indicate a contribution of motion to the recorded signals was detected.
Similar articles
- Electrical coupling between ventricular myocytes and myofibroblasts in the infarcted mouse heart.
Rubart M, Tao W, Lu XL, Conway SJ, Reuter SP, Lin SF, Soonpaa MH. Rubart M, et al. Cardiovasc Res. 2018 Mar 1;114(3):389-400. doi: 10.1093/cvr/cvx163. Cardiovasc Res. 2018. PMID: 29016731 Free PMC article. - Fibroblast-myocyte electrotonic coupling: does it occur in native cardiac tissue?
Kohl P, Gourdie RG. Kohl P, et al. J Mol Cell Cardiol. 2014 May;70(100):37-46. doi: 10.1016/j.yjmcc.2013.12.024. Epub 2014 Jan 8. J Mol Cell Cardiol. 2014. PMID: 24412581 Free PMC article. Review. - Fibroblast-myocyte coupling in the heart: Potential relevance for therapeutic interventions.
Ongstad E, Kohl P. Ongstad E, et al. J Mol Cell Cardiol. 2016 Feb;91:238-46. doi: 10.1016/j.yjmcc.2016.01.010. Epub 2016 Jan 14. J Mol Cell Cardiol. 2016. PMID: 26774702 Free PMC article. Review. - Electrical consequences of cardiac myocyte: fibroblast coupling.
McArthur L, Chilton L, Smith GL, Nicklin SA. McArthur L, et al. Biochem Soc Trans. 2015 Jun;43(3):513-8. doi: 10.1042/BST20150035. Biochem Soc Trans. 2015. PMID: 26009200 Review. - Sensing Cardiac Electrical Activity With a Cardiac Myocyte--Targeted Optogenetic Voltage Indicator.
Chang Liao ML, de Boer TP, Mutoh H, Raad N, Richter C, Wagner E, Downie BR, Unsöld B, Arooj I, Streckfuss-Bömeke K, Döker S, Luther S, Guan K, Wagner S, Lehnart SE, Maier LS, Stühmer W, Wettwer E, van Veen T, Morlock MM, Knöpfel T, Zimmermann WH. Chang Liao ML, et al. Circ Res. 2015 Aug 14;117(5):401-12. doi: 10.1161/CIRCRESAHA.117.306143. Epub 2015 Jun 15. Circ Res. 2015. PMID: 26078285
Cited by
- Progress of Conductivity and Conduction Velocity Measured in Human and Animal Hearts.
Fu Z, Dong R, Zheng H, Wang Z, Cao B, Bai J, Ma M, Song Z, Pan F, Xia L, Wu Y, Zhou S, Deng D. Fu Z, et al. Rev Cardiovasc Med. 2024 Oct 11;25(10):364. doi: 10.31083/j.rcm2510364. eCollection 2024 Oct. Rev Cardiovasc Med. 2024. PMID: 39484125 Free PMC article. Review. - Glucocorticoids preserve the t-tubular system in ventricular cardiomyocytes by upregulation of autophagic flux.
Seidel T, Fiegle DJ, Baur TJ, Ritzer A, Nay S, Heim C, Weyand M, Milting H, Oakley RH, Cidlowski JA, Volk T. Seidel T, et al. Basic Res Cardiol. 2019 Oct 31;114(6):47. doi: 10.1007/s00395-019-0758-6. Basic Res Cardiol. 2019. PMID: 31673803 Free PMC article. - Cardiac resident macrophages: key regulatory mediators in the aftermath of myocardial infarction.
Chen C, Wang J, Liu C, Hu J. Chen C, et al. Front Immunol. 2023 Jun 30;14:1207100. doi: 10.3389/fimmu.2023.1207100. eCollection 2023. Front Immunol. 2023. PMID: 37457720 Free PMC article. Review. - Synthetic recovery of impulse propagation in myocardial infarction via silicon carbide semiconductive nanowires.
Lagonegro P, Rossi S, Salvarani N, Lo Muzio FP, Rozzi G, Modica J, Bigi F, Quaretti M, Salviati G, Pinelli S, Alinovi R, Catalucci D, D'Autilia F, Gazza F, Condorelli G, Rossi F, Miragoli M. Lagonegro P, et al. Nat Commun. 2022 Jan 10;13(1):6. doi: 10.1038/s41467-021-27637-2. Nat Commun. 2022. PMID: 35013167 Free PMC article. - Cardiac Optogenetics: 2018.
Boyle PM, Karathanos TV, Trayanova NA. Boyle PM, et al. JACC Clin Electrophysiol. 2018 Feb;4(2):155-167. doi: 10.1016/j.jacep.2017.12.006. Epub 2018 Feb 1. JACC Clin Electrophysiol. 2018. PMID: 29749932 Free PMC article. Review.
References
- Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65(1):40–51. - PubMed
- Adler CP, Ringlage WP, Böhm N. [DNA content and cell number in heart and liver of children. Comparable biochemical, cytophotometric and histological investigations (author’s transl)] Pathol Res Pract. 1981;172(1-2):25–41. - PubMed
- Kohl P, Camelliti P. Fibroblast-myocyte connections in the heart. Heart Rhythm. 2012;9(3):461–464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FS/12/17/29532/BHF_/British Heart Foundation/United Kingdom
- FS/15/3/31047/BHF_/British Heart Foundation/United Kingdom
- P41 GM103431/GM/NIGMS NIH HHS/United States
- MOP-142424/CIHR/Canada
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous