Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial - PubMed (original) (raw)
Clinical Trial
. 2017 Jan 7;389(10064):67-76.
doi: 10.1016/S0140-6736(16)32455-2. Epub 2016 Dec 8.
Matthew D Galsky 2, Jonathan E Rosenberg 3, Thomas Powles 4, Daniel P Petrylak 5, Joaquim Bellmunt 6, Yohann Loriot 7, Andrea Necchi 8, Jean Hoffman-Censits 9, Jose Luis Perez-Gracia 10, Nancy A Dawson 11, Michiel S van der Heijden 12, Robert Dreicer 13, Sandy Srinivas 14, Margitta M Retz 15, Richard W Joseph 16, Alexandra Drakaki 17, Ulka N Vaishampayan 18, Srikala S Sridhar 19, David I Quinn 20, Ignacio Durán 21, David R Shaffer 22, Bernhard J Eigl 23, Petros D Grivas 24, Evan Y Yu 25, Shi Li 26, Edward E Kadel 3rd 26, Zachary Boyd 26, Richard Bourgon 26, Priti S Hegde 26, Sanjeev Mariathasan 26, AnnChristine Thåström 26, Oyewale O Abidoye 26, Gregg D Fine 26, Dean F Bajorin 3; IMvigor210 Study Group
Affiliations
- PMID: 27939400
- PMCID: PMC5568632
- DOI: 10.1016/S0140-6736(16)32455-2
Clinical Trial
Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial
Arjun V Balar et al. Lancet. 2017.
Erratum in
- Department of Error.
[No authors listed] [No authors listed] Lancet. 2017 Aug 26;390(10097):848. doi: 10.1016/S0140-6736(17)32213-4. Epub 2017 Aug 11. Lancet. 2017. PMID: 28807535 No abstract available.
Abstract
Background: First-line chemotherapy for patients with cisplatin-ineligible locally advanced or metastatic urothelial carcinoma is associated with short response duration, poor survival, and high toxicity. This study assessed atezolizumab (anti-programmed death-ligand 1 [PD-L1]) as treatment for metastatic urothelial cancer in cisplatin-ineligible patients.
Methods: For this single-arm, multicentre, phase 2 study, in 47 academic medical centres and community oncology practices in seven countries in North America and Europe, we recruited previously untreated patients with locally advanced or metastatic urothelial cancer who were cisplatin ineligible. Patients were given 1200 mg intravenous atezolizumab every 21 days until progression. The primary endpoint was independently confirmed objective response rate per Response Evaluation Criteria in Solid Tumors version 1.1 (central review), assessed in prespecified subgroups based on PD-L1 expression and in all patients. All participants who received one or more doses of atezolizumab were included in the primary and safety analyses. This study was registered with ClinicalTrials.gov, number NCT02108652.
Findings: Between June 9, 2014, and March 30, 2015, we enrolled 123 patients, of whom 119 received one or more doses of atezolizumab. At 17·2 months' median follow-up, the objective response rate was 23% (95% CI 16 to 31), the complete response rate was 9% (n=11), and 19 of 27 responses were ongoing. Median response duration was not reached. Responses occurred across all PD-L1 and poor prognostic factor subgroups. Median progression-free survival was 2·7 months (2·1 to 4·2). Median overall survival was 15·9 months (10·4 to not estimable). Tumour mutation load was associated with response. Treatment-related adverse events that occurred in 10% or more of patients were fatigue (36 [30%] patients), diarrhoea (14 [12%] patients), and pruritus (13 [11%] patients). One treatment-related death (sepsis) occurred. Nine (8%) patients had an adverse event leading to treatment discontinuation. Immune-mediated events occurred in 14 (12%) patients.
Interpretation: Atezolizumab showed encouraging durable response rates, survival, and tolerability, supporting its therapeutic use in untreated metastatic urothelial cancer.
Funding: F Hoffmann-La Roche, Genentech.
Trial registration: ClinicalTrials.gov NCT02302807 NCT02807636 NCT02108652.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
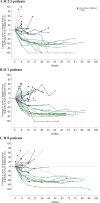
Figure 1. Change from baseline tumour burden by PD-L1 status
Spider plots depict changes from baseline tumour burden, defined as the sum of target lesion diameters, in patients who received atezolizumab, based on baseline PD-L1 status on immune cells of (A) IC2/3, (B) IC1, and (C) IC0. Grey colour denotes progressive disease; blue denotes stable disease; green denotes complete and partial responses (per RECIST v1.1). Data cutoff: July 4, 2016. PD-L1=programmed death-ligand 1. IC=tumour-infiltrating immune cell.
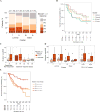
Figure 2. Overall survival in patients treated with atezolizumab
Kaplan-Meier estimates of overall survival according to PD-L1 status on immune cells. A total of 59 events occurred in all patients by the data cutoff date (18 in IC2/3 patients; 41 in IC0/1 patients). Data cutoff: July 4, 2016. PD-L1=programmed death-ligand 1. NE=not estimable. IC=tumour-infiltrating immune cell.
Figure 3. Associations among TCGA subtype, mutation load, and clinical activity
(A) Response as a function of The Cancer Genome Atlas subtype. (B) Kaplan-Meier plot of overall survival by subtype (luminal I, papillary-like; luminal II; basal III, squamous-like; and basal IV). (C) Mutation load as a function of response (Wilcoxon rank sum p=0·0180 for responding vs non-responding patients). (D) Mutation load versus response disaggregated by subtype or PD-L1 IC score. (E) Kaplan-Meier estimate of overall survival according to estimated mutation load (per megabase), binned into quartiles (log-rank p=0·0041 for a difference in overall survival between quartiles 1 to 3 and quartile 4). P values are for descriptive purposes only. TCGA=The Cancer Genome Atlas. PD-L1=programmed death-ligand 1. Data cutoff: July 4, 2016. IC=tumour-infiltrating immune cell. Lum=luminal. Bas=basal. RECIST=Response Evaluation Criteria In Solid Tumors. NE=not estimable. PD=progressive disease. SD=stable disease. PR=partial response. CR=complete response. MB=megabase.
Comment in
- Immune-checkpoint blockade in cisplatin-ineligible patients with urothelial cancer.
Faltas BM, Tagawa ST. Faltas BM, et al. Lancet. 2017 Jan 7;389(10064):6-7. doi: 10.1016/S0140-6736(16)32516-8. Epub 2016 Dec 8. Lancet. 2017. PMID: 27939401 No abstract available. - Urological cancer: Atezolizumab: an alternative to cisplatin?
Sidaway P. Sidaway P. Nat Rev Clin Oncol. 2017 Mar;14(3):139. doi: 10.1038/nrclinonc.2016.222. Epub 2016 Dec 29. Nat Rev Clin Oncol. 2017. PMID: 28031559 No abstract available. - Bladder cancer: Atezolizumab: an alternative to cisplatin?
Sidaway P. Sidaway P. Nat Rev Urol. 2017 Feb;14(2):67. doi: 10.1038/nrurol.2016.271. Epub 2016 Dec 29. Nat Rev Urol. 2017. PMID: 28031561 No abstract available.
Similar articles
- Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial.
Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, Srinivas S, Retz MM, Grivas P, Joseph RW, Galsky MD, Fleming MT, Petrylak DP, Perez-Gracia JL, Burris HA, Castellano D, Canil C, Bellmunt J, Bajorin D, Nickles D, Bourgon R, Frampton GM, Cui N, Mariathasan S, Abidoye O, Fine GD, Dreicer R. Rosenberg JE, et al. Lancet. 2016 May 7;387(10031):1909-20. doi: 10.1016/S0140-6736(16)00561-4. Epub 2016 Mar 4. Lancet. 2016. PMID: 26952546 Free PMC article. Clinical Trial. - First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study.
Balar AV, Castellano D, O'Donnell PH, Grivas P, Vuky J, Powles T, Plimack ER, Hahn NM, de Wit R, Pang L, Savage MJ, Perini RF, Keefe SM, Bajorin D, Bellmunt J. Balar AV, et al. Lancet Oncol. 2017 Nov;18(11):1483-1492. doi: 10.1016/S1470-2045(17)30616-2. Epub 2017 Sep 26. Lancet Oncol. 2017. PMID: 28967485 Clinical Trial. - Adjuvant atezolizumab versus observation in muscle-invasive urothelial carcinoma (IMvigor010): a multicentre, open-label, randomised, phase 3 trial.
Bellmunt J, Hussain M, Gschwend JE, Albers P, Oudard S, Castellano D, Daneshmand S, Nishiyama H, Majchrowicz M, Degaonkar V, Shi Y, Mariathasan S, Grivas P, Drakaki A, O'Donnell PH, Rosenberg JE, Geynisman DM, Petrylak DP, Hoffman-Censits J, Bedke J, Kalebasty AR, Zakharia Y, van der Heijden MS, Sternberg CN, Davarpanah NN, Powles T; IMvigor010 Study Group. Bellmunt J, et al. Lancet Oncol. 2021 Apr;22(4):525-537. doi: 10.1016/S1470-2045(21)00004-8. Epub 2021 Mar 12. Lancet Oncol. 2021. PMID: 33721560 Free PMC article. Clinical Trial. - [Atezolizumab (Tecentriq®): Activity, indication and modality of use in advanced or metastatic urinary bladder carcinoma].
Bernard-Tessier A, Bonnet C, Lavaud P, Gizzi M, Loriot Y, Massard C. Bernard-Tessier A, et al. Bull Cancer. 2018 Feb;105(2):140-145. doi: 10.1016/j.bulcan.2017.10.030. Epub 2017 Dec 28. Bull Cancer. 2018. PMID: 29290331 Review. French. - Atezolizumab in invasive and metastatic urothelial carcinoma.
Crist M, Balar A. Crist M, et al. Expert Rev Clin Pharmacol. 2017 Dec;10(12):1295-1301. doi: 10.1080/17512433.2017.1389275. Epub 2017 Oct 30. Expert Rev Clin Pharmacol. 2017. PMID: 28994323 Review.
Cited by
- Long-term survival following anti-PD-(L)1 monotherapy in advanced urothelial cancer and an assessment of potential prognostic clinical factors: a multicentre observational study.
Stockem CF, Einerhand SMH, Rodríguez IM, Salhi Y, Pérez E, Bakaloudi DR, Talukder R, Caramelo B, Morales-Barrera R, De Meulenaere A, Rametta A, Bottelli A, Lefort F, Giannatempo P, Vulsteke C, Carles J, Duran I, Grivas P, de Liaño AG, Robbrecht DGJ, Valderrama BP, van der Noort V, van der Heijden MS. Stockem CF, et al. BJC Rep. 2024 Oct 23;2(1):84. doi: 10.1038/s44276-024-00104-3. BJC Rep. 2024. PMID: 39516359 - Pan-Cancer Survival Impact of Immune Checkpoint Inhibitors in a National Healthcare System.
Miller SR, Schipper M, Fritsche LG, Jiang R, Strohbehn G, Ötleş E, McMahon BH, Crivelli S, Zamora-Resendiz R, Ramnath N, Yoo S, Dai X, Sankar K, Edwards DM, Allen SG, Green MD, Bryant AK. Miller SR, et al. Cancer Med. 2024 Nov;13(21):e70379. doi: 10.1002/cam4.70379. Cancer Med. 2024. PMID: 39508134 Free PMC article. - Anti-PD-1 immunotherapy for the treatment of metastatic urothelial carcinoma in a kidney transplant recipient: a case report.
Huang H, Dai Z, Jiang Z, Li X, Ma L, Ji Z, Fan X. Huang H, et al. BMC Nephrol. 2024 Oct 31;25(1):390. doi: 10.1186/s12882-024-03825-2. BMC Nephrol. 2024. PMID: 39482589 Free PMC article. - The prognostic value and immunological role of calcium/calmodulin dependent protein kinase kinase 2 (CAMKK2) in pan-cancer study.
Jin S, Wang Y, Hu S, Yan G. Jin S, et al. Medicine (Baltimore). 2024 Oct 11;103(41):e40072. doi: 10.1097/MD.0000000000040072. Medicine (Baltimore). 2024. PMID: 39465821 Free PMC article. - Blautia coccoides and its metabolic products enhance the efficacy of bladder cancer immunotherapy by promoting CD8+ T cell infiltration.
Wang B, Shangguan W, Li W, Xie M, Yu Y, Yang Q, Sun Q, Xue J, Zhu Z, Zhu Y, Wu P. Wang B, et al. J Transl Med. 2024 Oct 24;22(1):964. doi: 10.1186/s12967-024-05762-y. J Transl Med. 2024. PMID: 39449013 Free PMC article.
References
- National cancer institute surveillance, epidemiology, and end results program. SEER cancer statistics factsheets: Bladder cancer. [accessed August 12, 2016]; http://seer.cancer.gov/statfacts/html/urinb.html.
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase no. 11. [accessed September 13, 2016]; http://globocan.iarc.fr/Pages/summary_table_site_sel.aspx.
- Loehrer PJ, Sr, Einhorn LH, Elson PJ, et al. A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: A cooperative group study. J Clin Oncol. 1992;10:1066–73. - PubMed
- Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer "unfit" for cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–8. - PubMed
- Bellmunt J, Orsola A, Leow JJ, et al. Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(suppl 3):iii40–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials