Inhibition of human immunodeficiency virus type 1-mediated cytopathic effects by poly(L-lysine)-conjugated synthetic antisense oligodeoxyribonucleotides - PubMed (original) (raw)
Inhibition of human immunodeficiency virus type 1-mediated cytopathic effects by poly(L-lysine)-conjugated synthetic antisense oligodeoxyribonucleotides
M Stevenson et al. J Gen Virol. 1989 Oct.
Abstract
The ability of poly(L-lysine)-conjugated and methylphosphonate-modified synthetic human immunodeficiency virus type 1 (HIV-1) antisense oligodeoxyribonucleotides to protect susceptible host cells from the cytopathic effects of HIV-1 infection was studied. The abundance of viral antigens in oligomer-treated cultures indicated that the oligomers did not significantly affect viral infectivity. Similarly, no significant effects on relative viral RNA accumulation were apparent. The presence of poly(L-lysine)-modified oligomer complementary to the HIV-1 splice donor site resulted in a significant reduction in the production of viral structural proteins and virus titre in infected cultures. In addition, these cells were protected from HIV-1-mediated cytopathic effects while the other cultures rapidly succumbed to the cytotoxic effects of HIV-1 infection. The presence of poly(L-lysine)-conjugated oligomer resulted in the establishment of a persistent HIV-1 infection characterized by a highly productive virus infection in the absence of cell death while treatment of persistently infected cells with phorbol ester resulted in renewed cytopathicity. These results demonstrate the ability of synthetic antisense oligonucleotides to protect susceptible host cells from the cytopathic effects of HIV-1 infection.
Figures
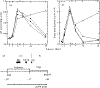
Fig 1.
Kinetics of viral replication in infected MT4 cells in the presence of antisense oligomers. MT4 cells were infected with HIV-1 and incubated with antisense oligomers as described in Methods. At the indicated times following infection, aliquots of culture supernatant and infected cells were removed for analysis of RT activity (a) and viral RNA levels by solution RNA hybridization (b) using single-stranded RNA probes generated from the LTR region of HIV-1 as shown in (c). +, Cells cultured without oligomer; ●, cells plus unmodified S.D. oligomer; ■, cells plus methylphosphonate-modified S.D. oligomer; ▲, cells plus poly(
l
-lysine)-modified S.D. oligomer.
Fig 2.
Inhibition of HIV-1 protein production in the presence of antisense oligomers. MT4 cells were infected and cultured with 1 μM parvovirus H-1 poly(
l
-lysine) oligomer (a); 200 nM-methylphosphonate-modified S.D. oligomer (b); and 200 nM-poly(
l
-lysine) S.D. oligomer (c). At 4 days p.i., cells were labelled for 3 h with [35S]methionine and [35S]cysteine and the viral proteins were immunoprecipitated with sera from a seronegative subject (lane 1) or sera from two HIV-1-seropositive asymptomatic haemophiliacs (lane 2,3) with titres of 5120 and 2560 respectively.
Fig. 3.
Southern blot analysis of HIV-1 DNA in persistently infected MT4 cells. Total cellular DNA was extracted from MT4 cells persistently infected with HIV-1, digested with Bgl_II, Hin_dIII, Kpn_I, Pvu_II_, Xba_I and _Eco_RI (lanes 1 to 6 respectively), blotted and hybridized with an HIV-1-specific genomic-length DNA probe.
Fig. 4.
Northern blot analysis of the CD4 mRNA levels in uninfected (lane 1) and persistently infected (lane 2) MT4 cells. Total cellular RNA (20 μg/lane) was denatured and electrophoresed through agarose gels containing formaldehyde and transferred to nylon membranes. The T4 probe was 3·0 kb cDNA pT4B (Maddon et al., 1986) containing the translated portion of the CD4 gene. _β_-Actin (Gunning et al., 1983) and _β_-tubulin (Hall et al., 1983) probes demonstrate equivalent amounts of RNA analysed in each lane.
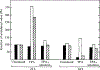
Fig. 5.
TPA-mediated stimulation of HIV-1 replication and cytotoxicity in persistently infected MT4 cells. Persistently infected cells were incubated with 1 ng/ml TPA plus α-amanitin (1 μg/ml) for 24 h followed by analysis of viability (trypan blue exclusion, shaded), RT (unshaded) and viral RNA (hatched) levels at 24 h and 84 h post-TP A addition. Values are expressed relative to those obtained from cultures incubated without TPA.
Similar articles
- Envelope glycoprotein of HIV induces interference and cytolysis resistance in CD4+ cells: mechanism for persistence in AIDS.
Stevenson M, Meier C, Mann AM, Chapman N, Wasiak A. Stevenson M, et al. Cell. 1988 May 6;53(3):483-96. doi: 10.1016/0092-8674(88)90168-7. Cell. 1988. PMID: 2966682 Free PMC article. - Persistent productive infection of human glial cells by human immunodeficiency virus (HIV) and by infectious molecular clones of HIV.
Dewhurst S, Sakai K, Bresser J, Stevenson M, Evinger-Hodges MJ, Volsky DJ. Dewhurst S, et al. J Virol. 1987 Dec;61(12):3774-82. doi: 10.1128/JVI.61.12.3774-3782.1987. J Virol. 1987. PMID: 2446007 Free PMC article. - Poly(L-lysine)-conjugated oligonucleotides promote sequence-specific inhibition of acute HIV-1 infection.
Degols G, Leonetti JP, Benkirane M, Devaux C, Lebleu B. Degols G, et al. Antisense Res Dev. 1992 Winter;2(4):293-301. doi: 10.1089/ard.1992.2.293. Antisense Res Dev. 1992. PMID: 1284042 - Studies on anti-human immunodeficiency virus oligonucleotides that have alternating methylphosphonate/phosphodiester linkages.
Miller PS, Cassidy RA, Hamma T, Kondo NS. Miller PS, et al. Pharmacol Ther. 2000 Mar;85(3):159-63. doi: 10.1016/s0163-7258(99)00054-6. Pharmacol Ther. 2000. PMID: 10739870 Review. - Oligonucleotides as inhibitors of human immunodeficiency virus.
Field AK. Field AK. Curr Opin Mol Ther. 1999 Jun;1(3):323-31. Curr Opin Mol Ther. 1999. PMID: 11713797 Review.
Cited by
- Synthesis, hybridization properties and antiviral activity of lipid-oligodeoxynucleotide conjugates.
Shea RG, Marsters JC, Bischofberger N. Shea RG, et al. Nucleic Acids Res. 1990 Jul 11;18(13):3777-83. doi: 10.1093/nar/18.13.3777. Nucleic Acids Res. 1990. PMID: 2165251 Free PMC article. - Hybridization properties of oligodeoxynucleotide pairs bridged by polyarginine peptides.
Wei Z, Tung CH, Zhu T, Dickerhof WA, Breslauer KJ, Georgopoulos DE, Leibowitz MJ, Stein S. Wei Z, et al. Nucleic Acids Res. 1996 Feb 15;24(4):655-61. doi: 10.1093/nar/24.4.655. Nucleic Acids Res. 1996. PMID: 8604306 Free PMC article. - Oligonucleotides with novel, cationic backbone substituents: aminoethylphosphonates.
Fathi R, Huang Q, Coppola G, Delaney W, Teasdale R, Krieg AM, Cook AF. Fathi R, et al. Nucleic Acids Res. 1994 Dec 11;22(24):5416-24. doi: 10.1093/nar/22.24.5416. Nucleic Acids Res. 1994. PMID: 7816633 Free PMC article. - Inhibition of HIV-LTR gene expression by oligonucleotides targeted to the TAR element.
Vickers T, Baker BF, Cook PD, Zounes M, Buckheit RW Jr, Germany J, Ecker DJ. Vickers T, et al. Nucleic Acids Res. 1991 Jun 25;19(12):3359-68. doi: 10.1093/nar/19.12.3359. Nucleic Acids Res. 1991. PMID: 2062653 Free PMC article.
References
- AGRIS CH, BLAKE KR, MILLER PS, REDDY MP & TS’O POP (1986). Inhibition of vesicular stomatitis virus protein synthesis and infection by sequence-specific oligodeoxyribonucleoside methylphosphonates. Biochemistry 25, 6268–6275. - PubMed
- BARRÉ-SINOUSSI F, CHERMAN JC, REY R, NUGEYERE MT, CHAMARET S, GRUEST J, DAUGUET C, AXLER-BLIN C, BRUN-VEXINET F, ROUZIOUX C, ROZENBAUM W & MONTAGNIER L (1983). Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immunodeficiency syndrome (AIDS). Science 220, 868–871. - PubMed
- CASAREALE D, DEWHURST S, SONNABEND J, SINANGIL F, PURTILO D & VOLSKY DJ (1985). Prevalence of AIDS-associated retrovirus and anti-bodies among male homosexuals at risk for AIDS in Greenwich Village. AIDS Research 1, 407–421. - PubMed
- CASAREALE D, STEVENSON M, SAKAI K & VOLSKY DJ (1987). A human T-cell line resistant to cytopathic effects of the human immunodeficiency virus (HIV-1). Virology 156, 40–49. - PubMed
- CHIRGWIN JM, PRYZBYLA AE, MCDONALD RJ & RUTTER WJ (1979). Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18, 5294–5299. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources