Therapeutic Strategies to Attenuate Hemorrhagic Transformation After Tissue Plasminogen Activator Treatment for Acute Ischemic Stroke - PubMed (original) (raw)
Review
. 2017 Mar 1;24(3):240-253.
doi: 10.5551/jat.RV16006. Epub 2016 Dec 13.
Affiliations
- PMID: 27980241
- PMCID: PMC5383539
- DOI: 10.5551/jat.RV16006
Review
Therapeutic Strategies to Attenuate Hemorrhagic Transformation After Tissue Plasminogen Activator Treatment for Acute Ischemic Stroke
Masato Kanazawa et al. J Atheroscler Thromb. 2017.
Abstract
This review focuses on the mechanisms and emerging concepts of stroke and therapeutic strategies for attenuating hemorrhagic transformation (HT) after tissue plasminogen activator (tPA) treatment for acute ischemic stroke (AIS). The therapeutic time window for tPA treatment has been extended. However, the patients who are eligible for tPA treatment are still <5% of all patients with AIS. The risk of serious or fatal symptomatic hemorrhage increases with delayed initiation of treatment. HT is thought to be caused by 1) ischemia/reperfusion injury; 2) the toxicity of tPA itself; 3) inflammation; and/or 4) remodeling factor-mediated effects. Modulation of these pathophysiologies is the basis of direct therapeutic strategies to attenuate HT after tPA treatment. Several studies have revealed that matrix metalloproteinases and free radicals are potential therapeutic targets. In addition, we have demonstrated that the inhibition of the vascular endothelial growth factor-signaling pathway and supplemental treatment with a recombinant angiopoietin-1 protein might be a promising therapeutic strategy for attenuating HT after tPA treatment through vascular protection. Moreover, single-target therapies could be insufficient for attenuating HT after tPA treatment and improving the therapeutic outcome of patients with AIS. We recently identified progranulin, which is a growth factor and a novel target molecule with multiple therapeutic effects. Progranulin might be a therapeutic target that protects the brain through suppression of vascular remodeling (vascular protection), neuroinflammation, and/or neuronal death (neuroprotection). Clinical trials which evaluate the effects of anti-VEGF drugs or PGRN-based treatment with tPA will be might worthwhile.
Conflict of interest statement
TS is an academic adviser of the ShimoJani LLC biotech company.
Figures
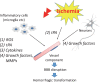
Fig. 1.
Mechanisms of intracerebral hemorrhagic transformation after tPA treatment and therapeutic targets BBB, blood-brain barrier; MMP, matrix metalloproteinase; ROS, reactive oxygen species; tPA, tissue plasminogen activator
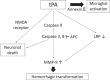
Fig. 2.
The cascade of tPA-induced adverse effects APC, activated protein C; LRP, Low-density lipoprotein receptor-related protein; MMP, matrix metalloproteinase; NMDA, N-methyl-D-aspartate; tPA, tissue plasminogen activator
Fig. 3.
The schema of protease-mediated BBB disruption BBB, blood-brain barrier; ECM, extracellular matrix; MMP, matrix metalloproteinase; tPA, tissue plasminogen activator
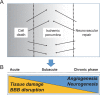
Fig. 4.
Ischemic penumbra (modified by reference 8) The new definition of ischemic penumbra is the transition region from injury to repair (A) and with time course after injury (B).
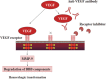
Fig. 5.
VEGF signaling cascade and anti-VEGF therapy (quoted from reference 57) After cerebral ischemia, vascular endothelial growth factor (VEGF) is expressed in the microvascular wall, and receptors that are conjugated to VEGF as a ligand are phosphorylated and activated. The subsequent activation of matrix metalloproteinase-9 (MMP-9) and degradation of protein components of the basal lamina cause intracerebral hemorrhage. The VEGF signaling cascade is inhibited by the anti-VEGF antibody that neutralizes VEGF and VEGF receptor inhibitors that inhibit VEGF receptors from being phosphorylated. BBB, blood-brain barrier.
Fig. 6.
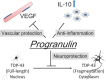
The pleiotropic effects of the brain protective target progranulin (PGRN) The growth factor PGRN could protect against acute focal cerebral ischemia through a variety of mechanisms, which we call brain protection. The nuclear protein TAR DNA binding protein-43 (TDP-43) is localized in the nucleus. However, cytoplasmic redistribution of TDP-43 occurs after ischemia. Intravenously administered recombinant PGRN significantly reduced the cerebral infarct and edema volumes, suppressed hemorrhagic transformation, and improved motor outcome in thromboembolic rats with delayed tissue plasminogen activator (tPA) treatment because of neuroprotection that occurred in part through the inhibition of the cytoplasmic redistribution of TDP-43, suppression of neuroinflammation through anti-inflammatory interleukin-10 (IL-10) in microglia, and attenuation of BBB disruption through the vascular endothelial growth factor (VEGF). PGRN might be a novel therapeutic target that provides brain protection through processes, such as vascular protection, anti-neuroinflammation, and neuroprotection.
Similar articles
- Strategies to prevent hemorrhagic transformation after reperfusion therapies for acute ischemic stroke: A literature review.
Otsu Y, Namekawa M, Toriyabe M, Ninomiya I, Hatakeyama M, Uemura M, Onodera O, Shimohata T, Kanazawa M. Otsu Y, et al. J Neurol Sci. 2020 Dec 15;419:117217. doi: 10.1016/j.jns.2020.117217. Epub 2020 Nov 4. J Neurol Sci. 2020. PMID: 33161301 Review. - Inhibition of VEGF signaling pathway attenuates hemorrhage after tPA treatment.
Kanazawa M, Igarashi H, Kawamura K, Takahashi T, Kakita A, Takahashi H, Nakada T, Nishizawa M, Shimohata T. Kanazawa M, et al. J Cereb Blood Flow Metab. 2011 Jun;31(6):1461-74. doi: 10.1038/jcbfm.2011.9. Epub 2011 Feb 9. J Cereb Blood Flow Metab. 2011. PMID: 21304556 Free PMC article. - NURR1 involvement in recombinant tissue-type plasminogen activator treatment complications after ischemic stroke.
Merino-Zamorano C, Hernández-Guillamon M, Jullienne A, Le Béhot A, Bardou I, Parés M, Fernández-Cadenas I, Giralt D, Carrera C, Ribó M, Vivien D, Ali C, Rosell A, Montaner J. Merino-Zamorano C, et al. Stroke. 2015 Feb;46(2):477-84. doi: 10.1161/STROKEAHA.114.006826. Epub 2014 Dec 11. Stroke. 2015. PMID: 25503547 - Iron Overload Exacerbates the Risk of Hemorrhagic Transformation After tPA (Tissue-Type Plasminogen Activator) Administration in Thromboembolic Stroke Mice.
García-Yébenes I, García-Culebras A, Peña-Martínez C, Fernández-López D, Díaz-Guzmán J, Negredo P, Avendaño C, Castellanos M, Gasull T, Dávalos A, Moro MA, Lizasoain I. García-Yébenes I, et al. Stroke. 2018 Sep;49(9):2163-2172. doi: 10.1161/STROKEAHA.118.021540. Stroke. 2018. PMID: 30018160 - Tissue-type plasminogen activator as a therapeutic target in stroke.
Gravanis I, Tsirka SE. Gravanis I, et al. Expert Opin Ther Targets. 2008 Feb;12(2):159-70. doi: 10.1517/14728222.12.2.159. Expert Opin Ther Targets. 2008. PMID: 18208365 Free PMC article. Review.
Cited by
- Macrophage Infiltration Reduces Neurodegeneration and Improves Stroke Recovery after Delayed Recanalization in Rats.
Pang J, Matei N, Peng J, Zheng W, Yu J, Luo X, Camara R, Chen L, Tang J, Zhang JH, Jiang Y. Pang J, et al. Oxid Med Cell Longev. 2022 Aug 17;2022:6422202. doi: 10.1155/2022/6422202. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 36035227 Free PMC article. - Effects of β-Adrenergic Blockade on Metabolic and Inflammatory Responses in a Rat Model of Ischemic Stroke.
Lin SY, Wang YY, Chang CY, Wu CC, Chen WY, Kuan YH, Liao SL, Chen CJ. Lin SY, et al. Cells. 2020 Jun 1;9(6):1373. doi: 10.3390/cells9061373. Cells. 2020. PMID: 32492962 Free PMC article. - Cerebral edema after ischemic stroke: Pathophysiology and underlying mechanisms.
Gu Y, Zhou C, Piao Z, Yuan H, Jiang H, Wei H, Zhou Y, Nan G, Ji X. Gu Y, et al. Front Neurosci. 2022 Aug 18;16:988283. doi: 10.3389/fnins.2022.988283. eCollection 2022. Front Neurosci. 2022. PMID: 36061592 Free PMC article. Review. - Combination therapy for ischemic stroke: Novel approaches to lengthen therapeutic window of tissue plasminogen activator.
Knecht T, Borlongan C, Dela Peña I. Knecht T, et al. Brain Circ. 2018 Jul-Sep;4(3):99-108. doi: 10.4103/bc.bc_21_18. Epub 2018 Oct 9. Brain Circ. 2018. PMID: 30450415 Free PMC article. Review. - Improving Cerebral Blood Flow after Arterial Recanalization: A Novel Therapeutic Strategy in Stroke.
El Amki M, Wegener S. El Amki M, et al. Int J Mol Sci. 2017 Dec 9;18(12):2669. doi: 10.3390/ijms18122669. Int J Mol Sci. 2017. PMID: 29232823 Free PMC article. Review.
References
- Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB, American Heart Association Statistics Committee; Stroke Statistics Subcommittee : Executive Summary: Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association: Circulation, 2016; 133: e38-360 - PubMed
- Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, Albers GW, Kaste M, Marler JR, Hamilton SA, Tilley BC, Davis SM, Donnan GA, Hacke W, ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group. Allen K, Mau J, Meier D, del Zoppo G, De Silva DA, Butcher KS, Parsons MW, Barber PA, Levi C, Bladin C, Byrnes G. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet, 2010; 375: 1695-1703 - PubMed
- IST-3 collaborative group. Sandercock P, Wardlaw JM, Lindley RI, Dennis M, Cohen G, Murray G, Innes K, Venables G, Czlonkowska A, Kobayashi A, Ricci S, Murray V, Berge E, Slot KB, Hankey GJ, Correia M, Peeters A, Matz K, Lyrer P, Gubitz G, Phillips SJ, Arauz A: The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet, 2012; 379: 2352-2363 - PMC - PubMed
- Whiteley WN, Emberson J, Lees KR, Blackwell L, Albers G, Bluhmki E, Brott T, Cohen G, Davis S, Donnan G, Grotta J, Howard G, Kaste M, Koga M, von Kummer R, Lansberg MG, Lindley RI, Lyden P, Olivot JM, Parsons M, Toni D, Toyoda K, Wahlgren N, Wardlaw J, Del Zoppo GJ, Sandercock P, Hacke W, Baigent C, Stroke Thrombolysis Trialists' Collaboration : Risk of intracerebral haemorrhage with alteplase after acute ischaemic stroke: a secondary analysis of an individual patient data meta-analysis. Lancet Neurol, 2016; 15: 925-933 - PubMed
- Kastrup A, Groschel K, Ringer TM, Redcker C, Cordesmeyer R, Witte OW, Terborg C: Early disruption of the blood-brain barrier after thrombolytic therapy predicts hemorrhage in patients with acute stroke. Stroke, 2008; 39: 2385-2387 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical