Regulation of Ketone Body Metabolism and the Role of PPARα - PubMed (original) (raw)
Review
Regulation of Ketone Body Metabolism and the Role of PPARα
Maja Grabacka et al. Int J Mol Sci. 2016.
Abstract
Ketogenesis and ketolysis are central metabolic processes activated during the response to fasting. Ketogenesis is regulated in multiple stages, and a nuclear receptor peroxisome proliferator activated receptor α (PPARα) is one of the key transcription factors taking part in this regulation. PPARα is an important element in the metabolic network, where it participates in signaling driven by the main nutrient sensors, such as AMP-activated protein kinase (AMPK), PPARγ coactivator 1α (PGC-1α), and mammalian (mechanistic) target of rapamycin (mTOR) and induces hormonal mediators, such as fibroblast growth factor 21 (FGF21). This work describes the regulation of ketogenesis and ketolysis in normal and malignant cells and briefly summarizes the positive effects of ketone bodies in various neuropathologic conditions.
Keywords: 3-hydroxy-3-methylglytaryl-CoA synthetase 2 (HMGCS2); fasting; fenofibrate; glioma; melanoma; β hydroxybutyrate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
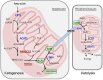
Figure 1
The metabolism of ketone bodies: ketogenesis takes place in hepatocyte mitochondria, whereas ketolysis involves utilization of ketone bodies in the mitochondria of peripheral tissues. ACAT1—acetoacetyl-CoA thiolase, Ac-CoA—acetyl-CoA, AcAc-CoA—acetoacetyl-CoA, BDH—β-hydroxybutyrate dehydrogenase, bHB—β-hydroxybutyrate, CPT1—carnitine palmitoyltransferase 1, HMGCL—HMG-CoA lyase, HMGCS2—HMG-CoA synthetase, MCT1—monocarboxylate transporter 1, SCOT—succinyl-CoA:3-ketocid-CoA transferase, TCA—tricarboxylic acid cycle.
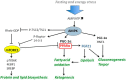
Figure 2
AMPK and mTOR complex 1 (mTORC1) respond to nutrient supply and cellular energy status. AMPK stimulates catabolism and ketogenesis through activation of PPARα and PGC-1α. mTORC1 blocks PPARα and induces anabolic processes, such as protein and lipid biosynthesis. The abbreviations are explained in the text.
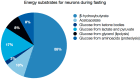
Figure 3
Energy substrates for brain during fasting. The values indicate the percentage of energy derived from utilization of each substrate.
Similar articles
- mTORC1 controls fasting-induced ketogenesis and its modulation by ageing.
Sengupta S, Peterson TR, Laplante M, Oh S, Sabatini DM. Sengupta S, et al. Nature. 2010 Dec 23;468(7327):1100-4. doi: 10.1038/nature09584. Nature. 2010. PMID: 21179166 - Human HMGCS2 regulates mitochondrial fatty acid oxidation and FGF21 expression in HepG2 cell line.
Vilà-Brau A, De Sousa-Coelho AL, Mayordomo C, Haro D, Marrero PF. Vilà-Brau A, et al. J Biol Chem. 2011 Jun 10;286(23):20423-30. doi: 10.1074/jbc.M111.235044. Epub 2011 Apr 18. J Biol Chem. 2011. PMID: 21502324 Free PMC article. - Acetylcholinesterase Inhibitor Donepezil Effects on Plasma β-Hydroxybutyrate Levels in the Treatment of Alzheimer's Disease.
Wan L, Lu J, Fu J, Huang J, Yang Q, Xin B, Chen L, Huo Y, Zhong Y, Guo C. Wan L, et al. Curr Alzheimer Res. 2018;15(10):917-927. doi: 10.2174/1567205015666180601091818. Curr Alzheimer Res. 2018. PMID: 29852870 - Regulation of energy metabolism by long-chain fatty acids.
Nakamura MT, Yudell BE, Loor JJ. Nakamura MT, et al. Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review. - Peroxisome proliferator activated receptor α ligands as anticancer drugs targeting mitochondrial metabolism.
Grabacka M, Pierzchalska M, Reiss K. Grabacka M, et al. Curr Pharm Biotechnol. 2013;14(3):342-56. doi: 10.2174/1389201011314030009. Curr Pharm Biotechnol. 2013. PMID: 21133850 Free PMC article. Review.
Cited by
- Ketogenesis alleviates TNFα-induced apoptosis and inflammatory responses in intestinal cells.
Kim JT, Napier DL, Kim J, Li C, Lee EY, Weiss HL, Wang Q, Evers BM. Kim JT, et al. Free Radic Biol Med. 2021 Aug 20;172:90-100. doi: 10.1016/j.freeradbiomed.2021.05.032. Epub 2021 Jun 1. Free Radic Biol Med. 2021. PMID: 34087430 Free PMC article. - Molecular Mechanisms for Ketone Body Metabolism, Signaling Functions, and Therapeutic Potential in Cancer.
Hwang CY, Choe W, Yoon KS, Ha J, Kim SS, Yeo EJ, Kang I. Hwang CY, et al. Nutrients. 2022 Nov 21;14(22):4932. doi: 10.3390/nu14224932. Nutrients. 2022. PMID: 36432618 Free PMC article. Review. - Ketosis Suppression and Ageing (KetoSAge): The Effects of Suppressing Ketosis in Long Term Keto-Adapted Non-Athletic Females.
Cooper ID, Kyriakidou Y, Edwards K, Petagine L, Seyfried TN, Duraj T, Soto-Mota A, Scarborough A, Jacome SL, Brookler K, Borgognoni V, Novaes V, Al-Faour R, Elliott BT. Cooper ID, et al. Int J Mol Sci. 2023 Oct 26;24(21):15621. doi: 10.3390/ijms242115621. Int J Mol Sci. 2023. PMID: 37958602 Free PMC article. - βOHB Protective Pathways in Aralar-KO Neurons and Brain: An Alternative to Ketogenic Diet.
Pérez-Liébana I, Casarejos MJ, Alcaide A, Herrada-Soler E, Llorente-Folch I, Contreras L, Satrústegui J, Pardo B. Pérez-Liébana I, et al. J Neurosci. 2020 Nov 25;40(48):9293-9305. doi: 10.1523/JNEUROSCI.0711-20.2020. Epub 2020 Oct 21. J Neurosci. 2020. PMID: 33087477 Free PMC article. - Mechanisms of Metabolic Reprogramming in Cancer Cells Supporting Enhanced Growth and Proliferation.
Schiliro C, Firestein BL. Schiliro C, et al. Cells. 2021 Apr 29;10(5):1056. doi: 10.3390/cells10051056. Cells. 2021. PMID: 33946927 Free PMC article. Review.
References
- Veech R.L. The therapeutic implications of ketone bodies: The effects of ketone bodies in pathological conditions: Ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism. Prostaglandins Leukot. Essent. Fat. Acids. 2004;70:309–319. doi: 10.1016/j.plefa.2003.09.007. - DOI - PubMed
- Alberti K.G., Johnston D.G., Gill A., Barnes A.J., Orskov H. Hormonal regulation of ketone-body metabolism in man. Biochem. Soc. Symp. 1978;43:163–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous