Motor Dysfunctions and Neuropathology in Mouse Models of Spinocerebellar Ataxia Type 2: A Comprehensive Review - PubMed (original) (raw)
Review
Motor Dysfunctions and Neuropathology in Mouse Models of Spinocerebellar Ataxia Type 2: A Comprehensive Review
João M Da Conceição Alves-Cruzeiro et al. Front Neurosci. 2016.
Abstract
Spinocerebellar ataxia type 2 (SCA2) is an autosomal dominant ataxia caused by an expansion of CAG repeats in the exon 1 of the gene ATXN2, conferring a gain of toxic function that triggers the appearance of the disease phenotype. SCA2 is characterized by several symptoms including progressive gait ataxia and dysarthria, slow saccadic eye movements, sleep disturbances, cognitive impairments, and psychological dysfunctions such as insomnia and depression, among others. The available treatments rely on palliative care, which mitigate some of the major symptoms but ultimately fail to block the disease progression. This persistent lack of effective therapies led to the development of several models in yeast, C. elegans, D. melanogaster, and mice to serve as platforms for testing new therapeutic strategies and to accelerate the research on the complex disease mechanisms. In this work, we review 4 transgenic and 1 knock-in mouse that exhibit a SCA2-related phenotype and discuss their usefulness in addressing different scientific problems. The knock-in mice are extremely faithful to the human disease, with late onset of symptoms and physiological levels of mutant ataxin-2, while the other transgenic possess robust and well-characterized motor impairments and neuropathological features. Furthermore, a new BAC model of SCA2 shows promise to study the recently explored role of non-coding RNAs as a major pathogenic mechanism in this devastating disorder. Focusing on specific aspects of the behavior and neuropathology, as well as technical aspects, we provide a highly practical description and comparison of all the models with the purpose of creating a useful resource for SCA2 researchers worldwide.
Keywords: knock-in; motor impairments; mouse; neuropathology; spinocerebellar ataxia type 2; transgenic.
Figures
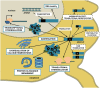
Figure 1
Molecular mechanisms proposed to be involved in SCA2 pathogenesis. The anti-sense transcription of the ATXN2 gene gives origin to the repeat-expanded ATXN2-AS, with the ability to form hairpin structures and induce toxicity. The sense transcription encodes to the polyQ-expanded ataxin-2 protein, which assembles in insoluble, not always ubiquitinated cytoplasmic aggregates, and recruits other proteins like E3 ubiquitin ligases. Ultimately, the UPS might get overburden, disturbing the neuronal protein turnover and engaging in aberrant proteolytic cleavage, with the formation of N-terminal PolyQ-containing toxic fragments. Also, the mutated ataxin-2 decreases the ability of WT ataxin-2 to stabilize mRNAs and upregulate protein expression, resulting in transcriptional and translational dysregulations. On the other hand, the expanded polyQ protein binds to receptors in the ER and promotes a significant increase in intracellular calcium and, consequently, excitotoxicity, and enhanced LTD in cerebellar PCs. Finally, posttranslational modifications such as phosphorylation at specific residues might modulate the toxicity of ataxin-2, and RAN translation can result in additional polyalanine and/or polyserine toxic proteins. Blue, solid line boxes represent well-studied disease mechanisms, with extensive supporting evidence in SCA2. Black, dashed line boxes represent pathogenic mechanisms that are well established in other PolyQ disorders like HD and other SCAs, but whose relevance to SCA2 is still unclear.
Similar articles
- Frequency of SCA1, SCA2, SCA3/MJD, SCA6, SCA7, and DRPLA CAG trinucleotide repeat expansion in patients with hereditary spinocerebellar ataxia from Chinese kindreds.
Tang B, Liu C, Shen L, Dai H, Pan Q, Jing L, Ouyang S, Xia J. Tang B, et al. Arch Neurol. 2000 Apr;57(4):540-4. doi: 10.1001/archneur.57.4.540. Arch Neurol. 2000. PMID: 10768629 - Generation of an Atxn2-CAG100 knock-in mouse reveals N-acetylaspartate production deficit due to early Nat8l dysregulation.
Sen NE, Canet-Pons J, Halbach MV, Arsovic A, Pilatus U, Chae WH, Kaya ZE, Seidel K, Rollmann E, Mittelbronn M, Meierhofer D, De Zeeuw CI, Bosman LWJ, Gispert S, Auburger G. Sen NE, et al. Neurobiol Dis. 2019 Dec;132:104559. doi: 10.1016/j.nbd.2019.104559. Epub 2019 Jul 31. Neurobiol Dis. 2019. PMID: 31376479 - ATXN2-AS, a gene antisense to ATXN2, is associated with spinocerebellar ataxia type 2 and amyotrophic lateral sclerosis.
Li PP, Sun X, Xia G, Arbez N, Paul S, Zhu S, Peng HB, Ross CA, Koeppen AH, Margolis RL, Pulst SM, Ashizawa T, Rudnicki DD. Li PP, et al. Ann Neurol. 2016 Oct;80(4):600-15. doi: 10.1002/ana.24761. Ann Neurol. 2016. PMID: 27531668 Free PMC article. - Spinocerebellar Ataxia Type 2: Clinicogenetic Aspects, Mechanistic Insights, and Management Approaches.
Velázquez-Pérez LC, Rodríguez-Labrada R, Fernandez-Ruiz J. Velázquez-Pérez LC, et al. Front Neurol. 2017 Sep 11;8:472. doi: 10.3389/fneur.2017.00472. eCollection 2017. Front Neurol. 2017. PMID: 28955296 Free PMC article. Review. - [Advance in research on spinocerebellar ataxia 2].
Jing F, Yang D, Chen T. Jing F, et al. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2018 Apr 10;35(2):284-287. doi: 10.3760/cma.j.issn.1003-9406.2018.02.032. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2018. PMID: 29653012 Review. Chinese.
Cited by
- Post-symptomatic administration of hMSCs exerts therapeutic effects in SCA2 mice.
Kim S, Sharma C, Hong J, Kim JH, Nam Y, Kim MS, Lee TY, Kim KS, Suk K, Lee HW, Kim SR. Kim S, et al. Stem Cell Res Ther. 2024 Nov 9;15(1):411. doi: 10.1186/s13287-024-04020-8. Stem Cell Res Ther. 2024. PMID: 39521966 Free PMC article. - Gene Deregulation and Underlying Mechanisms in Spinocerebellar Ataxias With Polyglutamine Expansion.
Niewiadomska-Cimicka A, Hache A, Trottier Y. Niewiadomska-Cimicka A, et al. Front Neurosci. 2020 Jun 9;14:571. doi: 10.3389/fnins.2020.00571. eCollection 2020. Front Neurosci. 2020. PMID: 32581696 Free PMC article. Review. - Ataxin-2: a powerful RNA-binding protein.
Li L, Wang M, Huang L, Zheng X, Wang L, Miao H. Li L, et al. Discov Oncol. 2024 Jul 22;15(1):298. doi: 10.1007/s12672-024-01158-y. Discov Oncol. 2024. PMID: 39039334 Free PMC article. Review. - FAM98A is localized to stress granules and associates with multiple stress granule-localized proteins.
Ozeki K, Sugiyama M, Akter KA, Nishiwaki K, Asano-Inami E, Senga T. Ozeki K, et al. Mol Cell Biochem. 2019 Jan;451(1-2):107-115. doi: 10.1007/s11010-018-3397-6. Epub 2018 Jul 10. Mol Cell Biochem. 2019. PMID: 29992460 - Consensus Paper: Strengths and Weaknesses of Animal Models of Spinocerebellar Ataxias and Their Clinical Implications.
Cendelin J, Cvetanovic M, Gandelman M, Hirai H, Orr HT, Pulst SM, Strupp M, Tichanek F, Tuma J, Manto M. Cendelin J, et al. Cerebellum. 2022 Jun;21(3):452-481. doi: 10.1007/s12311-021-01311-1. Epub 2021 Aug 10. Cerebellum. 2022. PMID: 34378174 Free PMC article.
References
- Aguiar J., Fernández J., Aguilar A., Mendoza Y., Vázquez M., Suárez J., et al. . (2006). Ubiquitous expression of human SCA2 gene under the regulation of the SCA2 self promoter cause specific Purkinje cell degeneration in transgenic mice. Neurosci. Lett. 392, 202–206. 10.1016/j.neulet.2005.09.020 - DOI - PubMed
- Babovic-Vuksanovic D., Snow K., Patterson M. C., Michels V. V. (1998). Spinocerebellar ataxia type 2 (SCA 2) in an infant with extreme CAG repeat expansion. Am. J. Med. Genet. 79, 383–387. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials