Clinical Validation of Reduced Alcohol Consumption After Treatment for Alcohol Dependence Using the World Health Organization Risk Drinking Levels - PubMed (original) (raw)
Randomized Controlled Trial
. 2017 Jan;41(1):179-186.
doi: 10.1111/acer.13272. Epub 2016 Dec 26.
Affiliations
- PMID: 28019652
- PMCID: PMC5205540
- DOI: 10.1111/acer.13272
Randomized Controlled Trial
Clinical Validation of Reduced Alcohol Consumption After Treatment for Alcohol Dependence Using the World Health Organization Risk Drinking Levels
Katie Witkiewitz et al. Alcohol Clin Exp Res. 2017 Jan.
Abstract
Background: Alcohol use disorder (AUD) is a highly prevalent public health problem associated with considerable individual and societal costs. Abstinence from alcohol is the most widely accepted target of treatment for AUD, but it severely limits treatment options and could deter individuals who prefer to reduce their drinking from seeking treatment. Clinical validation of reduced alcohol consumption as the primary outcome of alcohol clinical trials is critical for expanding treatment options. One potentially useful measure of alcohol treatment outcome is a reduction in the World Health Organization (WHO, International Guide for Monitoring Alcohol Consumption and Related Harm. Geneva, Switzerland, 2000) risk levels of alcohol use (very high risk, high risk, moderate risk, and low risk). For example, a 2-shift reduction in WHO risk levels (e.g., high risk to low risk) has been used by the European Medicines Agency (2010, Guideline on the Development of Medicinal Products for the Treatment of Alcohol Dependence. UK) to evaluate nalmefene as a treatment for alcohol dependence (AD; Mann et al. 2013, Biol Psychiatry 73, 706-13).
Methods: The current study was a secondary data analysis of the COMBINE study (n = 1,383; Anton et al., ) to examine the association between reductions in WHO risk levels and reductions in alcohol-related consequences and mental health symptoms during and following treatment in patients with AD.
Results: Any reduction in WHO risk drinking level during treatment was associated with significantly fewer alcohol-related consequences and improved mental health at the end of treatment and for up to 1 year posttreatment. A greater reduction in WHO risk drinking level predicted a greater reduction in consequences and greater improvements in mental health.
Conclusions: Changes in WHO risk levels appear to be a valid end point for alcohol clinical trials. Based on the current findings, reductions in WHO risk drinking levels during treatment reflect meaningful reductions in alcohol-related consequences and improved functioning.
Keywords: Alcohol Dependence; Alcohol Treatment Outcomes; Harm Reduction; Reduced Alcohol Consumption; World Health Organization Risk Drinking Levels.
Copyright © 2016 by the Research Society on Alcoholism.
Figures
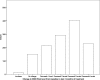
Figure 1
Histogram (count) of individuals who had an increase, no change, or decrease in WHO risk level from baseline to the end of treatment.
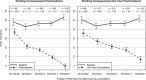
Figure 2
Average Drinker Inventory of Consequences (DrInC) total scores by change in WHO risk level from baseline (solid line) to the end of treatment and posttreatment (dashed lines). Vertical bars indicate 95% confidence intervals (CIs). n = number of participants with data available for analysis within each level of WHO risk change. d = Cohen's d effect size, computed as the difference in means from baseline to follow‐up within each level of WHO risk change divided by the standard deviation at baseline within the same level of WHO risk change. All means and 95% CIs (baseline and follow‐up) were estimated using linear regression and controlled for age at baseline, gender, race, education, body mass index at baseline, and smoker status at baseline; follow‐up estimates also controlled for baseline values of the dependent variable and baseline WHO risk level. All control variables were grand‐mean‐centered.
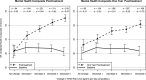
Figure 3
Average 12‐item Short Form Health Survey (SF‐12) Mental Health Composite Scores by change in WHO risk level from baseline (solid line) to the end of treatment and posttreatment (dashed lines). Vertical bars indicate 95% confidence intervals (CIs). n = number of participants with data available for analysis within each level of WHO risk change. d = Cohen's d effect size, computed as the difference in means from baseline to follow‐up within each level of WHO risk change divided by the standard deviation at baseline within the same level of WHO risk change. All means and 95% CIs (baseline and follow‐up) were estimated using linear regression and controlled for age at baseline, gender, race, education, body mass index at baseline, and smoker status at baseline; follow‐up estimates also controlled for baseline values of the dependent variable and baseline WHO risk level. All control variables were grand‐mean‐centered.
Comment in
- Toward Rational, Evidence-Based, and Clinically Relevant Measures to Determine Improvement Following Treatment for Alcohol Use Disorder.
Johnson BA. Johnson BA. Alcohol Clin Exp Res. 2017 Apr;41(4):703-707. doi: 10.1111/acer.13341. Epub 2017 Feb 25. Alcohol Clin Exp Res. 2017. PMID: 28118502 No abstract available. - Letter to Editor in Response to Johnson's Commentary (2017) on the Witkiewitz and Colleagues (2017) Article.
Litten RZ, Falk DE, O'Malley SS, Witkiewitz KA, Mann KF, Anton RF. Litten RZ, et al. Alcohol Clin Exp Res. 2017 Jul;41(7):1381-1382. doi: 10.1111/acer.13411. Epub 2017 May 23. Alcohol Clin Exp Res. 2017. PMID: 28471501 Free PMC article. No abstract available. - FDA and EMA Need Homology on Alcohol Outcome Measures-Semper: Simplicitas est purius modum.
Johnson BA. Johnson BA. Alcohol Clin Exp Res. 2017 Jul;41(7):1383-1384. doi: 10.1111/acer.13412. Epub 2017 May 29. Alcohol Clin Exp Res. 2017. PMID: 28471508 No abstract available.
Similar articles
- World Health Organization risk drinking level reductions are associated with improved functioning and are sustained among patients with mild, moderate and severe alcohol dependence in clinical trials in the United States and United Kingdom.
Witkiewitz K, Heather N, Falk DE, Litten RZ, Hasin DS, Kranzler HR, Mann KF, O'Malley SS, Anton RF. Witkiewitz K, et al. Addiction. 2020 Sep;115(9):1668-1680. doi: 10.1111/add.15011. Epub 2020 Mar 10. Addiction. 2020. PMID: 32056311 Free PMC article. Clinical Trial. - Maintenance of World Health Organization Risk Drinking Level Reductions and Posttreatment Functioning Following a Large Alcohol Use Disorder Clinical Trial.
Witkiewitz K, Falk DE, Litten RZ, Hasin DS, Kranzler HR, Mann KF, O'Malley SS, Anton RF. Witkiewitz K, et al. Alcohol Clin Exp Res. 2019 May;43(5):979-987. doi: 10.1111/acer.14018. Epub 2019 Apr 5. Alcohol Clin Exp Res. 2019. PMID: 30951210 Free PMC article. Clinical Trial. - Drinking Risk Level Reductions Associated with Improvements in Physical Health and Quality of Life Among Individuals with Alcohol Use Disorder.
Witkiewitz K, Kranzler HR, Hallgren KA, O'Malley SS, Falk DE, Litten RZ, Hasin DS, Mann KF, Anton RF. Witkiewitz K, et al. Alcohol Clin Exp Res. 2018 Dec;42(12):2453-2465. doi: 10.1111/acer.13897. Epub 2018 Nov 5. Alcohol Clin Exp Res. 2018. PMID: 30395350 Free PMC article. Clinical Trial. - [Benefits in reducing alcohol consumption: how nalmefene can help].
Bendimerad P, Blecha L. Bendimerad P, et al. Encephale. 2014 Dec;40(6):495-500. doi: 10.1016/j.encep.2014.10.012. Epub 2014 Nov 22. Encephale. 2014. PMID: 25454365 Review. French. - Risks and Benefits of Nalmefene in the Treatment of Adult Alcohol Dependence: A Systematic Literature Review and Meta-Analysis of Published and Unpublished Double-Blind Randomized Controlled Trials.
Palpacuer C, Laviolle B, Boussageon R, Reymann JM, Bellissant E, Naudet F. Palpacuer C, et al. PLoS Med. 2015 Dec 22;12(12):e1001924. doi: 10.1371/journal.pmed.1001924. eCollection 2015 Dec. PLoS Med. 2015. PMID: 26694529 Free PMC article. Review.
Cited by
- Non-abstinent treatment outcomes for cannabis use disorder.
Levin FR, Mariani JJ, Choi CJ, Basaraba C, Brooks DJ, Brezing CA, Pavlicova M. Levin FR, et al. Drug Alcohol Depend. 2021 Aug 1;225:108765. doi: 10.1016/j.drugalcdep.2021.108765. Epub 2021 May 21. Drug Alcohol Depend. 2021. PMID: 34087745 Free PMC article. - Hazardous drinking and alcohol use disorders.
MacKillop J, Agabio R, Feldstein Ewing SW, Heilig M, Kelly JF, Leggio L, Lingford-Hughes A, Palmer AA, Parry CD, Ray L, Rehm J. MacKillop J, et al. Nat Rev Dis Primers. 2022 Dec 22;8(1):80. doi: 10.1038/s41572-022-00406-1. Nat Rev Dis Primers. 2022. PMID: 36550121 Free PMC article. Review. - World Health Organization risk drinking level reductions are associated with improved functioning and are sustained among patients with mild, moderate and severe alcohol dependence in clinical trials in the United States and United Kingdom.
Witkiewitz K, Heather N, Falk DE, Litten RZ, Hasin DS, Kranzler HR, Mann KF, O'Malley SS, Anton RF. Witkiewitz K, et al. Addiction. 2020 Sep;115(9):1668-1680. doi: 10.1111/add.15011. Epub 2020 Mar 10. Addiction. 2020. PMID: 32056311 Free PMC article. Clinical Trial. - Evaluating cannabis use risk reduction as an alternative clinical outcome for cannabis use disorder.
Sherman BJ, Sofis MJ, Borodovsky JT, Gray KM, McRae-Clark AL, Budney AJ. Sherman BJ, et al. Psychol Addict Behav. 2022 Aug;36(5):505-514. doi: 10.1037/adb0000760. Epub 2021 Jul 1. Psychol Addict Behav. 2022. PMID: 34197135 Free PMC article. Clinical Trial. - SF-6D utility scores for alcohol use disorder status and alcohol consumption risk levels in the US population.
Barbosa C, Bray JW, Dowd WN, Barnosky A, Wittenberg E. Barbosa C, et al. Addiction. 2021 May;116(5):1034-1042. doi: 10.1111/add.15224. Epub 2020 Aug 27. Addiction. 2021. PMID: 33448504 Free PMC article.
References
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 295:2003–2017. - PubMed
- Aubin H‐J, Daeppen J‐B (2013) Emerging pharmacotherapies for alcohol dependence: a systematic review focusing on reduction in consumption. Drug Alcohol Depend 133:15–29. - PubMed
- Aubin H‐J, Reimer J, Nutt DJ, Bladström A, Torup L, François C, Chick J (2015) Clinical relevance of as‐needed treatment with nalmefene in alcohol‐dependent patients. Eur Addict Res 21:160–168. - PubMed
- Bouchery EE, Harwood HJ, Sacks JJ, Simon CJ, Brewer RD (2011) Economic costs of excessive alcohol consumption in the US, 2006. Am J Prev Med 41:516–524. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AA022328/AA/NIAAA NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- T32 AA007455/AA/NIAAA NIH HHS/United States
- P50 AA010761/AA/NIAAA NIH HHS/United States
- R01 AA025309/AA/NIAAA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical