Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application - PubMed (original) (raw)
Review
. 2017 Feb 28;8(9):16052-16074.
doi: 10.18632/oncotarget.14109.
Young Kwang Chae 1 2 3, Peter S Hammerman 4, Christos Vaklavas 5, Nisha Mohindra 1 2 3, Aparna Kalyan 1 2 3, Maria Matsangou 1 2 3, Ricardo Costa 1, Benedito Carneiro 1 2 3, Victoria M Villaflor 1 2 3, Massimo Cristofanilli 1 2 3, Francis J Giles 1 2 3
Affiliations
- PMID: 28030802
- PMCID: PMC5362545
- DOI: 10.18632/oncotarget.14109
Review
Inhibition of the fibroblast growth factor receptor (FGFR) pathway: the current landscape and barriers to clinical application
Young Kwang Chae et al. Oncotarget. 2017.
Abstract
The fibroblast growth factor/fibroblast growth factor receptor (FGF/FGFR) is a tyrosine kinase signaling pathway that has a fundamental role in many biologic processes including embryonic development, tissue regeneration, and angiogenesis. Increasing evidence indicates that this pathway plays a critical role in oncogenesis via gene amplification, activating mutations, or translocation in tumors of various histologies. With multiplex sequencing technology, the detection of FGFR aberrations has become more common and is tied to cancer cell proliferation, resistance to anticancer therapies, and neoangiogenesis. Inhibition of FGFR signaling appears promising in preclinical studies, suggesting a pathway of clinical interest in the development of targeted therapy. Phase I trials have demonstrated a manageable toxicity profile. Currently, there are multiple FGFR inhibitors under study with many non-selective (multi-kinase) inhibitors demonstrating limited clinical responses. As we progress from the first generation of non-selective drugs to the second generation of selective FGFR inhibitors, it is clear that FGFR aberrations do not behave uniformly across cancer types; thus, a deeper understanding of biomarker strategies is undoubtedly warranted. This review aims to consolidate data from recent clinical trials with a focus on selective FGFR inhibitors. As Phase II clinical trials emerge, concentration on patient selection as it pertains to predicting response to therapy, feasible methods for overcoming toxicity, and the likelihood of combination therapies should be utilized. We will also discuss qualities that may be desirable in future generations of FGFR inhibitors, with the hope that overcoming these current barriers will expedite the availability of this novel class of medications.
Keywords: factor; fibroblast; growth; inhibition; receptor.
Conflict of interest statement
Conflicts of interest
No relevant conflicts of interests to report.
Figures
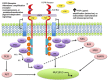
Figure 1. Molecular aberrations leading to FGFR pathway activation
The FGFRs dimerize upon ligand binding and trigger a downstream cascade of signaling pathways. The FGFR receptors (1-4) can become activated by mutation, translocation, or gene amplification. An increase in circulating FGF ligands can also cause activation. Downstream signaling can trigger the mitogen activated protein kinase (MAPK) pathway, the phosphoinositide-3-kinase (PI3K/Akt) pathway, the phosphorylation of the signal transducer and activator of transcription (STAT), and the PLCγ activation of the DAG-PKC and IP3-Ca2+ cascade resulting in DNA transcription. Negative feedback loops can attenuate the signaling cascade at varying levels. As seen above, the “similar expression to FGF” (SEF) family members can interact with the cytoplasmic domain of FGFRs and inhibit downstream signaling. It is hypothesized that FGFRL1 (atypical receptor/FGFR5) may serve as a ligand trap, may dimerize with other transmembrane FGFRs and inhibit autophosphorylation, or may increase turnover rates of other FGFRs [16]. No evidence exists for these mechanisms.
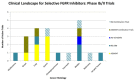
Figure 2. Selected overview of phase II clinical trials evaluating FGFR Inhibitors as monotherapy or in combination with existing therapies
Bladder, Lung, and Gastric cancers all are areas of interest in advanced clinical trials testing FGFR pathway inhibition. There exist a wide scope of histologies where FGFR inhibition may be of clinical benefit, many phase II trials are currently in the recruiting stages or are actively ongoing.
Similar articles
- Selective FGFR/FGF pathway inhibitors: inhibition strategies, clinical activities, resistance mutations, and future directions.
Repetto M, Crimini E, Giugliano F, Morganti S, Belli C, Curigliano G. Repetto M, et al. Expert Rev Clin Pharmacol. 2021 Oct;14(10):1233-1252. doi: 10.1080/17512433.2021.1947246. Expert Rev Clin Pharmacol. 2021. PMID: 34591728 Review. - Fibroblast Growth Factor (FGF) Receptor/FGF Inhibitors: Novel Targets and Strategies for Optimization of Response of Solid Tumors.
Hierro C, Rodon J, Tabernero J. Hierro C, et al. Semin Oncol. 2015 Dec;42(6):801-19. doi: 10.1053/j.seminoncol.2015.09.027. Epub 2015 Sep 24. Semin Oncol. 2015. PMID: 26615127 Review. - Targeting Drugs Against Fibroblast Growth Factor(s)-Induced Cell Signaling.
Agrawal S, Maity S, AlRaawi Z, Al-Ameer M, Kumar TKS. Agrawal S, et al. Curr Drug Targets. 2021;22(2):214-240. doi: 10.2174/1389450121999201012201926. Curr Drug Targets. 2021. PMID: 33045958 Review. - Targeting FGFR Signaling in Cancer.
Touat M, Ileana E, Postel-Vinay S, André F, Soria JC. Touat M, et al. Clin Cancer Res. 2015 Jun 15;21(12):2684-94. doi: 10.1158/1078-0432.CCR-14-2329. Clin Cancer Res. 2015. PMID: 26078430 Review. - Recent developments and advances of FGFR as a potential target in cancer.
Xue WJ, Li MT, Chen L, Sun LP, Li YY. Xue WJ, et al. Future Med Chem. 2018 Sep 1;10(17):2109-2126. doi: 10.4155/fmc-2018-0103. Epub 2018 Aug 1. Future Med Chem. 2018. PMID: 30066580 Review.
Cited by
- Complete Response to a Fibroblast Growth Factor Receptor Inhibitor in a Patient With Head and Neck Squamous Cell Carcinoma Harboring FGF Amplifications.
Dumbrava EI, Alfattal R, Miller VA, Tsimberidou AM. Dumbrava EI, et al. JCO Precis Oncol. 2018;2:PO.18.00100. doi: 10.1200/PO.18.00100. Epub 2018 Aug 31. JCO Precis Oncol. 2018. PMID: 31123723 Free PMC article. No abstract available. - FGFR1β is a driver isoform of FGFR1 alternative splicing in breast cancer cells.
Zhao M, Zhuo ML, Zheng X, Su X, Meric-Bernstam F. Zhao M, et al. Oncotarget. 2019 Jan 1;10(1):30-44. doi: 10.18632/oncotarget.26530. eCollection 2019 Jan 1. Oncotarget. 2019. PMID: 30713601 Free PMC article. - FGFR- gene family alterations in low-grade neuroepithelial tumors.
Bale TA. Bale TA. Acta Neuropathol Commun. 2020 Feb 21;8(1):21. doi: 10.1186/s40478-020-00898-6. Acta Neuropathol Commun. 2020. PMID: 32085805 Free PMC article. Review. - Metastatic Calcinosis Cutis Secondary to Selective Fibroblast Growth Factor Receptor Inhibitor: Rapid and Complete Regression after Blood Phosphate Normalization and Drug Withdrawal.
Lopez-Castillo D, March-Rodriguez A, Rodriguez-Vida A, Pujol RM, Segura S. Lopez-Castillo D, et al. Acta Derm Venereol. 2020 Mar 12;100(6):adv00079. doi: 10.2340/00015555-3438. Acta Derm Venereol. 2020. PMID: 32115665 Free PMC article. No abstract available. - Transposon Insertion Mutagenesis in Mice for Modeling Human Cancers: Critical Insights Gained and New Opportunities.
Beckmann PJ, Largaespada DA. Beckmann PJ, et al. Int J Mol Sci. 2020 Feb 10;21(3):1172. doi: 10.3390/ijms21031172. Int J Mol Sci. 2020. PMID: 32050713 Free PMC article. Review.
References
- Neilson KM, Friesel R. Ligand-independent activation of fibroblast growth factor receptors by point mutations in the extracellular, transmembrane, and kinase domains. J Biol Chem. 1996;271(40):25049–25057. - PubMed
- Becker D, Lee PL, Rodeck U, Herlyn M. Inhibition of the fibroblast growth factor receptor 1 (FGFR-1) gene in human melanocytes and malignant melanomas leads to inhibition of proliferation and signs indicative of differentiation. Oncogene. 1992;7(11):2303–2313. - PubMed
- Funato N, Moriyama K, Shimokawa H, Kuroda T. Basic fibroblast growth factor induces apoptosis in myofibroblastic cells isolated from rat palatal mucosa. Biochem Biophys Res Commun. 1997;240(1):21–26. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials