Influenza virus exploits tunneling nanotubes for cell-to-cell spread - PubMed (original) (raw)
Jin Hyang Kim 1, Priya Ranjan 1, Maureen G Metcalfe 2, Weiping Cao 1, Margarita Mishina 1, Shivaprakash Gangappa 1, Zhu Guo 3, Edward S Boyden 4, Sherif Zaki 2, Ian York 1, Adolfo García-Sastre 5, Michael Shaw 6, Suryaprakash Sambhara 1
Affiliations
- PMID: 28059146
- PMCID: PMC5216422
- DOI: 10.1038/srep40360
Influenza virus exploits tunneling nanotubes for cell-to-cell spread
Amrita Kumar et al. Sci Rep. 2017.
Abstract
Tunneling nanotubes (TNTs) represent a novel route of intercellular communication. While previous work has shown that TNTs facilitate the exchange of viral or prion proteins from infected to naïve cells, it is not clear whether the viral genome is also transferred via this mechanism and further, whether transfer via this route can result in productive replication of the infectious agents in the recipient cell. Here we present evidence that lung epithelial cells are connected by TNTs, and in spite of the presence of neutralizing antibodies and an antiviral agent, Oseltamivir, influenza virus can exploit these networks to transfer viral proteins and genome from the infected to naïve cell, resulting in productive viral replication in the naïve cells. These observations indicate that influenza viruses can spread using these intercellular networks that connect epithelial cells, evading immune and antiviral defenses and provide an explanation for the incidence of influenza infections even in influenza-immune individuals and vaccine failures.
Figures
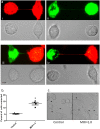
Figure 1. TNTs connect neighboring epithelial cells.
(a and b) TNT connecting neighboring A549 cells in a non-confluent monolayer (a) or a confluent monolayer (b) as imaged using the method of expansion microscopy. Lower panels show a higher magnification of the TNT at the cellular ends of the TNT shown in the upper panel. The scale bar corresponds to 10 μm. For both panels a and b, samples were chemically fixed, infused with polyelectrolyte gel, followed by dialysis in water, and image captured with a Zeiss confocal microscope equipped with a 100x/1.4 NA oil objective. (c) Ultra structure of TNTs. Scanning electron microscopy (SEM) shows the ultrastructure of a TNT between two A549 cells. Scale bar corresponds to 6.6 μm. (d) A TEM section of A549 cells cultured for 4 h after seeding, indicates the presence of TNTs connecting neighboring A549 cells. (e) Selected frames of a video sequence showing the dynamic and transient nature of TNTs connecting two A549 cells. Arrowhead highlights a TNT connecting two cells that bursts 45 sec after exposure to high laser power. (f) Mitochondria transfer via TNTs connecting two A549 cells. A549 cells were stained with Mitotracker Green or Mitotracker Red, mixed 1:1, and co-incubated for an additional 4 hrs before imaging. Green arrowheads highlight a red-stained mitochondria in cells stained with Mitotracker Green and red arrowheads highlight a green-stained mitochondria in cell stained with Mitotracker Red.
Figure 2. TNTs form between infected and uninfected A549 cells.
(a, panel i and ii) TNTs readily form between uninfected A549 cells labelled with membrane dye DiD (red), or between A549 cells infected with PR8 virus and labelled with DiO (Green). After 1 h of co-culture nanotubes formed by uninfected and infected A549 cells were assessed (Scale bars: 10 μm). (a, panel iii and iv) TNTs were also seen readily connecting neighboring A549 cells either uninfected (red) or PR8 infected (green) cells. Corresponding bright field images are also shown. (b and c) Panel showing increased frequency of TNT formation in A549 cells infected with PR8 virus when compared to uninfected cells. The frequency of TNT formation between uninfected and PR8 infected A549 cells is shown after 2–4 h of incubation at 37 °C (n = 500; number of cell counts over three independent experiments); difference between uninfected and infected cells is significant (t-test, P < 0.05).
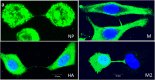
Figure 3. TNTs serve as a conduit for intracellular transport of IAV proteins.
Fluorescent images showing accumulation of influenza virus proteins NP, HA, M, and M1 proteins within TNTs. A population of A549 cells was infected with PR8 virus for 18 h, trypsinized and seeded on glass-bottom plates for 2 h after which they were imaged as described in materials and methods. Viral proteins are observed in the membrane nanotubes.
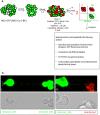
Figure 4. Two population model to evaluate spread of the viral proteins and genome via TNTs.
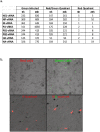
(a) Schematics of the two population model design and its application in tracking the fate of the recipient (uninfected) cells. Cells were sorted 6 h after infection with IAV expressing the NS1-GFP construct, mixed with an equal number of RFP-A459 cells, and co-incubated at 37 °C for an additional 4–6 h. The co-incubated cells were then sorted into populations expressing detectable RFP only (R-quadrant), GFP only (G-quadrant), or RFP plus GFP (i.e. initially-uninfected RFP-A549 cells that also expressed NS1-GFP) (R/G quadrant). Cells in these three quadrants were subjected to further analysis as described in the schematic. (b) Detection of endogenous NS1-GFP transfer in A549 cells through TNTs. NS1-GFP signal is seen as bright puncta in TNTs of A549 cells infected with NS1-GFP virus or in the TNTs connecting uninfected DiD-labelled-A549 cells (red) and NS1-GFP virus infected cells (green).
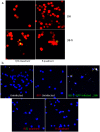
Figure 5. TNTs facilitate transfer of viral proteins and genome.
Cells of the R- and R/G- quadrant were cultured for up to 36 h post-sort and the images taken at the indicated time points post-sort. (a) Fluorescent images showing expression of NS1-GFP in the cells R/G- or the R-quadrant after incubation at 37 °C for an additional 36 h post-sorting. The cells were also subjected to in-situ RNA hybridization. (b) In situ RNA hybridization in cells of the R/G- or the R- quadrant.
Figure 6. Expression of viral genes in an uninfected population of cells co-cultured with influenza infected cells.
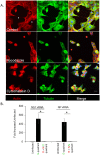
Cells of the R- and R/G- quadrant were cultured for up to 36 h post-sort and assays conducted as denoted. (a) RT-PCR analysis in cells of the R/G and R quadrant showing fold-increase in expression of viral genes. (b) Immunofluorescence-based infectivity assay showing GFP fluorescence in MDCK cells overlaid with cells from the R/G and R quadrants.
Figure 7. Pharmaceutical drugs that inhibit cytoskeletal dynamics attenuate the number of TNTs and cell-to-cell spread of the viral genome.
(A) A549 cells were incubated for 6 h with or without cytochalasin D and nocodozole, and stained for actin (Red) and Tubulin (Green) filaments before imaging and recording in 3D by confocal microscopy. Shown are the maximum intensity projections of the focal planes of a representative image stack from control and drug-treated cells. Arrows indicate TNTs observed in control (DMSO-treated) cells. TNTs were not observed in cells treated with either drug compared to the untreated-control. For each time point > 1000 cells were analyzed, and the experiment was repeated three times. Scale bar: 10 μm. (B) NS1-GFP infected cells were incubated with uninfected RFP-A549 cells, in the presence of neutralizing antibodies (2000 HI units), Oseltamivir (100 μM) and Cytochalasin D (20 μM). After 8 h of incubation, the cells in the R-and R/G-quadrants and G-quadrant were sorted. The sorted cells were cultured in the presence of Cytochalasin D (20 μM) for an additional 6 h, after which RNA was extracted and the expression of vRNA for NP and NS1 were analyzed using RT-PCR. The graph shows the expression of vRNA genes, NP and NS1 and data are shown as mean ± SD. β-Actin was used as a loading control. ANOVA was performed to compare the expression in cells in the G-quadrant versus cells in the R- and R/G-quadrants, and p values < 0.05 are indicated with an asterisk.
Similar articles
- Tunneling Nanotubes as a Novel Route of Cell-to-Cell Spread of Herpesviruses.
Panasiuk M, Rychłowski M, Derewońko N, Bieńkowska-Szewczyk K. Panasiuk M, et al. J Virol. 2018 Apr 27;92(10):e00090-18. doi: 10.1128/JVI.00090-18. Print 2018 May 15. J Virol. 2018. PMID: 29491165 Free PMC article. - Bridging the Gap: Virus Long-Distance Spread via Tunneling Nanotubes.
Jansens RJJ, Tishchenko A, Favoreel HW. Jansens RJJ, et al. J Virol. 2020 Mar 31;94(8):e02120-19. doi: 10.1128/JVI.02120-19. Print 2020 Mar 31. J Virol. 2020. PMID: 32024778 Free PMC article. Review. - Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells.
Lu J, Zheng X, Li F, Yu Y, Chen Z, Liu Z, Wang Z, Xu H, Yang W. Lu J, et al. Oncotarget. 2017 Feb 28;8(9):15539-15552. doi: 10.18632/oncotarget.14695. Oncotarget. 2017. PMID: 28107184 Free PMC article. - Porcine Reproductive and Respiratory Syndrome Virus Utilizes Nanotubes for Intercellular Spread.
Guo R, Katz BB, Tomich JM, Gallagher T, Fang Y. Guo R, et al. J Virol. 2016 Apr 29;90(10):5163-5175. doi: 10.1128/JVI.00036-16. Print 2016 May 15. J Virol. 2016. PMID: 26984724 Free PMC article. - A role for tunneling nanotubes in virus spread.
Lv W, Li Z, Wang S, He J, Zhang L. Lv W, et al. Front Microbiol. 2024 Feb 16;15:1356415. doi: 10.3389/fmicb.2024.1356415. eCollection 2024. Front Microbiol. 2024. PMID: 38435698 Free PMC article. Review.
Cited by
- Virus stealth technology: Tools to study virus cell-to-cell transmission.
Yin P, Martin CK, Kielian M. Yin P, et al. PLoS Pathog. 2024 Oct 9;20(10):e1012590. doi: 10.1371/journal.ppat.1012590. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39383183 Free PMC article. Review. No abstract available. - Invisible Bridges: Unveiling the Role and Prospects of Tunneling Nanotubes in Cancer Therapy.
Chen M, Zhao D. Chen M, et al. Mol Pharm. 2024 Nov 4;21(11):5413-5429. doi: 10.1021/acs.molpharmaceut.4c00563. Epub 2024 Oct 7. Mol Pharm. 2024. PMID: 39373242 Free PMC article. Review. - Intercellular Transport of Viral Proteins.
Simon F, Thoma-Kress AK. Simon F, et al. Results Probl Cell Differ. 2024;73:435-474. doi: 10.1007/978-3-031-62036-2_18. Results Probl Cell Differ. 2024. PMID: 39242389 Review. - Tunneling Nanotubes: The Cables for Viral Spread and Beyond.
Kapoor D, Sharma P, Saini A, Azhar E, Elste J, Kohlmeir EK, Shukla D, Tiwari V. Kapoor D, et al. Results Probl Cell Differ. 2024;73:375-417. doi: 10.1007/978-3-031-62036-2_16. Results Probl Cell Differ. 2024. PMID: 39242387 Review. - Mechanisms of Intracellular Communication in Cancer and Pathogen Spreading.
Valdebenito S, Ajasin D, Valerdi K, Liu YR, Rao S, Eugenin EA. Valdebenito S, et al. Results Probl Cell Differ. 2024;73:301-326. doi: 10.1007/978-3-031-62036-2_13. Results Probl Cell Differ. 2024. PMID: 39242384 Review.
References
- Sun X. & Whittaker G. R. Entry of influenza virus. Advances in experimental medicine and biology 790, 72–82 (2013). - PubMed
- Ohmit S. E., Petrie J. G., Cross R. T., Johnson E. & Monto A. S. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. The Journal of infectious diseases 204, 1879–1885 (2011). - PubMed
- Davis D. M. & Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nature reviews. Molecular cell biology 9, 431–436 (2008). - PubMed
- Onfelt B., Purbhoo M. A., Nedvetzki S., Sowinski S. & Davis D. M. Long-distance calls between cells connected by tunneling nanotubules. Science’s STKE: signal transduction knowledge environment 2005, pe55 (2005). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources