Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane - PubMed (original) (raw)
Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane
M H Barcellos-Hoff et al. Development. 1989 Feb.
Abstract
An essential feature of mammary gland differentiation during pregnancy is the formation of alveoli composed of polarized epithelial cells, which, under the influence of lactogenic hormones, secrete vectorially and sequester milk proteins. Previous culture studies have described either organization of cells polarized towards lumina containing little or no demonstrable tissue-specific protein, or establishment of functional secretory cells exhibiting little or no glandular architecture. In this paper, we report that tissue-specific vectorial secretion coincides with the formation of functional alveoli-like structures by primary mammary epithelial cells cultured on a reconstituted basement membrane matrix (derived from Engelbreth-Holm-Swarm murine tumour). Morphogenesis of these unique three-dimensional structures was initiated by cell-directed remodelling of the exogenous matrix leading to reorganization of cells into matrix-ensheathed aggregates by 24 h after plating. The aggregates subsequently cavitated, so that by day 6 the cells were organized into hollow spheres in which apical cell surfaces faced lumina sealed by tight junctions and basal surfaces were surrounded by a distinct basal lamina. The profiles of proteins secreted into the apical (luminal) and basal (medium) compartments indicated that these alveoli-like structures were capable of an appreciable amount of vectorial secretion. Immunoprecipitation with a broad spectrum milk antiserum showed that more than 80% of caseins were secreted into the lumina, whereas iron-binding proteins (both lactoferrin and transferrin) were present in comparable amounts in each compartment. Thus, these mammary cells established protein targeting pathways directing milk-specific proteins to the luminal compartment. A time course monitoring secretory activity demonstrated that establishment of tissue-specific vectorial secretion and increased total and milk protein secretion coincided with functional alveolar-like multicellular architecture. This culture system is unique among models of epithelial cell polarity in that it demonstrates several aspects of epithelial cell polarization: vectorial secretion, apical junctions, a sequestered compartment and formation of a basal lamina. These lumina-containing structures therefore reproduce the dual role of mammary epithelia to secrete vectorially and to sequester milk proteins. Thus, in addition to maintaining tissue-specific cytodifferentiation and function, a basement membrane promotes the expression of tissue-like morphogenesis.
Figures
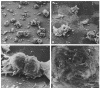
Fig. 1
Development of lumen-containing structures by cells cultured on EHS matrix. Isolated primary mammary epithelial cells were cultured on EHS matrix in the presence of lactogenic hormones for 3h (A), 24h (B), or 5 days (C,D), fixed, critical point dried and observed with SEM. The isolated epithelial cells are seeded as small clumps and scattered single cells that adhere rapidly to the matrix substratum by 3h (A), but do not appear to spread out on it. After 24h in culture (B), the cell clumps have attached firmly to the matrix and have apparently pulled it in toward themselves, leaving large areas of the culture dish cleared. By 4–6 days in culture (C), most of the cells are present in spheroids, which are completely surrounded by matrix. At higher magnification (D), the fibrillar nature of the matrix meshwork surrounding the bracketed spheroid in C is clearly seen. (A-C) Bar, 100_μ_m; (D) bar, 25_μ_m.
Fig. 2
Early ultrastructural organization of cells cultured on EHS (e). (A) Cross-section of a clump of epithelial cells 3h after plating shows that they are not intact mammary gland ducts or alveoli. The cells are unorganized and dead cells and cell debris (d) are scattered throughout the clumps. After two days in culture (b) lumen (lu) formation has begun, apparently by a process of cavitation within clumps. Cells surrounding the nascent lumina are not yet well oriented. Bar, 5_μ_m.
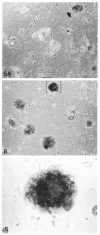
Fig. 3
Cross-section views of secretory alveolar-like structures. After culture on EHS matrix for 8 days, cells are organized into a variety of alveolar-like structures ranging from 50-150 _μ_m in diameter. The cells in these spheroids are polarized with their apices toward the lumen and their basal surfaces outward, contacting the basement membrane matrix. In some cases (A), the central lumen is quite small and filled with microvilli (arrow), while other (B) spheroids appear swollen with protein accumulated within their lumina. Likewise in A the cells are tall and columnar while in B the flattened cells are cuboidal. Individual cells in both of these structures show many morphological signs of secretory activity, including secretory granules, lipid droplets (L), and well-developed rough endoplasmic reticulum, although they are more prevalent in A. Bar, 10_μ_m.
Fig. 4
High-magnification view of secretory epithelial cell. In a section through an entire cell (A), many morphological indications of secretory activity are evident, including large indented basal nucleus (nu), multiple stacks of rough endoplasmic reticulum (rer), an elaborate Golgi complex (G), lipid droplets (L) and secretory vesicles in the apical cytoplasm, and numerous apical microvilli. These alveolar-like structures are characteristically sealed by tight and adherens junctions between cells near their apical borders (A,B, large arrows) and are completely surrounded by a distinct thin basal lamina (C, small arrows), apparently assembled by the cells during culture on the EHS matrix. (A) Bar, 1_μ_m; (B,C) bar, 0·5 _μ_m.
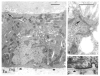
Fig. 5
Prevalence of lumen-containing structures in cultures on EHS matrix as demonstrated by phase-contrast microscopy after EGTA/trypan blue treatment. (A) Control culture exposed to trypan blue without EGTA; closed arrow designates dye-containing structure. (B) EGTA/trypan-blue-treated culture; open arrow designates dye-excluding structure; brackets designate the structure shown in C. (C) Higher magnification of bracketed structure in B containing a large trypan blue-containing lumen.
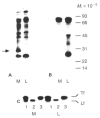
Fig. 6
Gel electrophoretic patterns of [35S]methionine-labelled proteins secreted into the medium and the luminal compartment by primary mammary epithelial cells cultured on plastic and EHS substrata for 6 days. (A) Unprecipitated proteins. Equal volumes of medium and EGTA extract were mixed 1:1 with sample buffer and run on 12·5% PAGE. The lanes in the fluorogram show medium (M) and lumina (L) compartments and position of molecular weight standards (_M_r × 10−3). Note the protein band of approximately 30×l03 _M_r (arrow) that is present only in the medium of the EHS cultures. (B) Immunoprecipitated milk proteins. Equal acid-precipitable counts from medium and luminal compartments were immunoprecipitated using a broad spectrum antibody to mouse skim milk proteins. (C) Specific immunoprecipitation of transferrin (Tf) and lactoferrin (Lf) by broad spectrum milk antibody (1), lactoferrin antibody (2), and transferrin antibody (3).
Fig. 7
Time course of DNA content and total protein secretion by cells cultured on EHS matrix. (A) DNA levels are expressed as a fraction of the amount of DNA plated at the initiation of culture (45 _μ_g dish−1). Closed symbols represent the mean of duplicate cultures on EHS matrix of two separate experiments. Open symbols represent the mean of duplicate cultures on plastic. (B) Total secretion by cells cultured on EHS matrix, as indicated by acid-preciptable counts (corrected for DNA content); symbols represent the mean of duplicate cultures; closed symbols represent secretion into medium, open symbols represent secretion into the luminal compartment.
Fig. 8
Time course of milk protein secretion into the medium and the EGTA-sensitive compartments by cells cultured on EHS matrix. (A) Proportion of _β_-casein in the medium (black bars) and lumina (open bars). The areas of the bands of interest were integrated by scanning densitometry and are expressed in arbitrary units; similar determinations for _α_-casein produced a comparable pattern (not shown). (B) Relative amounts of secreted α (squares) and _β_-casein (diamonds) in the medium (closed symbols) and lumina (open symbols) were determined by correcting the relative density of protein bands shown in A for total secretion and normalizing for DNA content. (C) Proportion of transferrin in the medium (black bars) and lumina (open bars) determined as in A. (D) Total amount of transferrin in the medium (closed symbols) and lumina (open symbols) determined as in B. These data were from the same experiment as represented in Fig. 7B.
Similar articles
- Vectorial secretion by constitutive and regulated secretory pathways in mammary epithelial cells.
Blatchford DR, Hendry KA, Turner MD, Burgoyne RD, Wilde CJ. Blatchford DR, et al. Epithelial Cell Biol. 1995;4(1):8-16. Epithelial Cell Biol. 1995. PMID: 8563795 - Cytodifferentiation of mouse mammary epithelial cells cultured on a reconstituted basement membrane reveals striking similarities to development in vivo.
Aggeler J, Ward J, Blackie LM, Barcellos-Hoff MH, Streuli CH, Bissell MJ. Aggeler J, et al. J Cell Sci. 1991 Jun;99 ( Pt 2):407-17. doi: 10.1242/jcs.99.2.407. J Cell Sci. 1991. PMID: 1885677 - Regulation of milk protein and basement membrane gene expression: the influence of the extracellular matrix.
Aggeler J, Park CS, Bissell MJ. Aggeler J, et al. J Dairy Sci. 1988 Oct;71(10):2830-42. doi: 10.3168/jds.S0022-0302(88)79879-3. J Dairy Sci. 1988. PMID: 3060493 Review. - Regulation of functional cytodifferentiation and histogenesis in mammary epithelial cells: role of the extracellular matrix.
Bissell MJ, Ram TG. Bissell MJ, et al. Environ Health Perspect. 1989 Mar;80:61-70. doi: 10.1289/ehp.898061. Environ Health Perspect. 1989. PMID: 2647485 Free PMC article. Review.
Cited by
- A Decision Tree to Guide Human and Mouse Mammary Organoid Model Selection.
Caruso M, Saberiseyedabad K, Mourao L, Scheele CLGJ. Caruso M, et al. Methods Mol Biol. 2024;2764:77-105. doi: 10.1007/978-1-0716-3674-9_7. Methods Mol Biol. 2024. PMID: 38393590 - The molecular basis of mammary gland development and epithelial differentiation.
Slepicka PF, Somasundara AVH, Dos Santos CO. Slepicka PF, et al. Semin Cell Dev Biol. 2021 Jun;114:93-112. doi: 10.1016/j.semcdb.2020.09.014. Epub 2020 Oct 17. Semin Cell Dev Biol. 2021. PMID: 33082117 Free PMC article. Review. - Deep nuclear invaginations are linked to cytoskeletal filaments - integrated bioimaging of epithelial cells in 3D culture.
Jorgens DM, Inman JL, Wojcik M, Robertson C, Palsdottir H, Tsai WT, Huang H, Bruni-Cardoso A, López CS, Bissell MJ, Xu K, Auer M. Jorgens DM, et al. J Cell Sci. 2017 Jan 1;130(1):177-189. doi: 10.1242/jcs.190967. Epub 2016 Aug 5. J Cell Sci. 2017. PMID: 27505896 Free PMC article. - Comparative growth of normal and malignant mouse mammary epithelium cultured serum-free on a biomatrix from preadipocytes.
Shappell NW, Lazo RO, Asch BB. Shappell NW, et al. In Vitro Cell Dev Biol. 1991 Jul;27A(7):569-77. doi: 10.1007/BF02631288. In Vitro Cell Dev Biol. 1991. PMID: 1716254 - Unraveling Heterogeneity in Epithelial Cell Fates of the Mammary Gland and Breast Cancer.
Samocha A, Doh H, Kessenbrock K, Roose JP. Samocha A, et al. Cancers (Basel). 2019 Sep 24;11(10):1423. doi: 10.3390/cancers11101423. Cancers (Basel). 2019. PMID: 31554261 Free PMC article. Review.
References
- Agoeler J, Park C, Bissell MJ. Regulation of milk protein and basement membrane gene expression: The influence of the extracellular matrix. J. Dairy Sci. 1988;71:2830–2842. - PubMed
- Barcellos-Hoff MH, Neville PN, Aggeler J, Bissell MJ. Polarized secretion by mammary epithelial cell cultures on EHS-matrix. J. Cell Biol. 1987;105:220a.
- Bissell MJ, Aggeler J. Dynamic reciprocity: How do extracellular matrix and hormones direct gene expression? In: Cabot MC, Mckeehan WL, editors. Mechanisms of Signal Transduction by Hormones and Growth Factors. Alan Liss; New York: 1987. pp. 251–262. - PubMed
- Bissell MJ, Hall HG. Form and function in the mammary gland: The role of extracellular matrix. In: Neville M, Daniel C, editors. The Mammary Gland: Development, Regulation and Function. Plenum Press Publishing Corporation; New York: 1987. pp. 97–146.
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA143836/CA/NCI NIH HHS/United States
- U54 CA126552/CA/NCI NIH HHS/United States
- 5T32 CA09272/CA/NCI NIH HHS/United States
- U54 CA112970/CA/NCI NIH HHS/United States
- R01 CA057621/CA/NCI NIH HHS/United States
- U01 CA143233/CA/NCI NIH HHS/United States
- R01 CA064786/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources