Cas13b Is a Type VI-B CRISPR-Associated RNA-Guided RNase Differentially Regulated by Accessory Proteins Csx27 and Csx28 - PubMed (original) (raw)
. 2017 Feb 16;65(4):618-630.e7.
doi: 10.1016/j.molcel.2016.12.023. Epub 2017 Jan 5.
David B T Cox 2, Neena K Pyzocha 3, Kaijie Zheng 4, Ian M Slaymaker 4, Jonathan S Gootenberg 5, Omar A Abudayyeh 6, Patrick Essletzbichler 4, Sergey Shmakov 7, Kira S Makarova 8, Eugene V Koonin 8, Feng Zhang 9
Affiliations
- PMID: 28065598
- PMCID: PMC5432119
- DOI: 10.1016/j.molcel.2016.12.023
Cas13b Is a Type VI-B CRISPR-Associated RNA-Guided RNase Differentially Regulated by Accessory Proteins Csx27 and Csx28
Aaron A Smargon et al. Mol Cell. 2017.
Abstract
CRISPR-Cas adaptive immune systems defend microbes against foreign nucleic acids via RNA-guided endonucleases. Using a computational sequence database mining approach, we identify two class 2 CRISPR-Cas systems (subtype VI-B) that lack Cas1 and Cas2 and encompass a single large effector protein, Cas13b, along with one of two previously uncharacterized associated proteins, Csx27 and Csx28. We establish that these CRISPR-Cas systems can achieve RNA interference when heterologously expressed. Through a combination of biochemical and genetic experiments, we show that Cas13b processes its own CRISPR array with short and long direct repeats, cleaves target RNA, and exhibits collateral RNase activity. Using an E. coli essential gene screen, we demonstrate that Cas13b has a double-sided protospacer-flanking sequence and elucidate RNA secondary structure requirements for targeting. We also find that Csx27 represses, whereas Csx28 enhances, Cas13b-mediated RNA interference. Characterization of these CRISPR systems creates opportunities to develop tools to manipulate and monitor cellular transcripts.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures
Figure 1. Discovery of two Class 2 CRISPR-Cas systems, subtype VI-B1 and VI-B2, containing Cas13b
(A) Bioinformatic pipeline to discover putative Class 2 CRISPR loci lacking Cas1 and Cas2. (B) A schematic phylogenetic tree of the subtype VI-B loci. Loci with Csx27 (brown) comprise variant VI-B1; loci with Csx28 (gold) comprise variant VI-B2. See also Figures S1, S2, and S3.
Figure 2. Cas13b from the VI-B1 locus processes a CRISPR array with two direct repeat variants
(A) RNA-Sequencing of the native VI-B1 locus from Bergeyella zoohelcum ATCC 43767. (B) Denaturing gel showing cleavage products of in vitro synthesized short-DR containing or long-DR containing CRISPR arrays from the B. zoohelcum genome by either wildtype or HEPN mutant BzCas13b (D1, R116A/H121A; D2, R1177A/H1182A; Q, R116A/H121A/R1177A/H1182A). The schematic shows fragment lengths of a cleaved CRISPR array. See also Figure S4 and Table S1.
Figure 3. Heterologous expression of Cas13b mediates knockdown of E. coli essential genes by a double-sided PFS
(A) Design of E. coli essential gene screen to determine targeting rules of nucleic acid interference. (B) Manhattan plots of mean spacer depletions mapped over 45 genes and aggregated across normalized gene distance for either the full B. zoohelcum VI-B1 locus (left) or cas13b alone (right), with non-targeting spacers in gray, safely depleted spacers (>5σ above mean depletion of non-targeting spacers) above blue line, and strongly depleted spacers (top 1% depleted) above red line. For the full locus, 36,142 targeting spacers and 630 non-targeting spacers passed QC filter. Of the targeting, 367 are strongly depleted and 1672 are safely depleted. For cas13b alone, 35,272 targeting spacers and 633 non-targeting spacers passed QC filter. Of the targeting, 359 are strongly depleted and 6374 are safely depleted. (C) Weblogo of sequence motifs of strongly depleted B. zoohelcum spacers. (D) Normalized PFS score matrix, where each score is the ratio of number of safely depleted B. zoohelcum spacers to total number of spacers for a given PFS, scaled so that maximum PFS score is 1. (E) Spacers targeting kanamycin to validate PFS targeting rules of 5′ PFS (D) and 3′ PFS (NAN or NNA). (F) Schematic of kanamycin validation screen for B. zoohelcum cas13b in E. coli. (G) Results from kanamycin validation screen; spacer abundances versus control for individual B. zoohelcum spacers, with abundances colored by type of spacer. See also Figure S5 and Tables S2, S3, S4, and S5.
Figure 4. Cas13b is a programmable single-stranded RNase with collateral activity
(A) Schematic showing the RNA secondary structure of the cleavage target in complex with a targeting 30-nt spacer connected to short direct repeat (top). Denaturing gel demonstrating short direct repeat and long direct repeat crRNA-mediated ssRNA cleavage (bottom). Reactions were incubated for 10 minutes. The ssRNA target is 5′ labeled with IRDye 800. Three cleavage sites are observed. (B) Schematic showing three numbered protospacers for each colored 5′ PFS on a body-labeled ssRNA target (top). Denaturing gel showing crRNA-guided ssRNA cleavage activity demonstrating the requirement for a D 5′ PFS (not C) (bottom). Reactions were incubated for 60 minutes. crRNAs correspond to protospacer numbered from the 5′ to the 3′ end of the target. Gel lane containing RNA ladder not shown. (C) Schematic of a body-labeled ssRNA substrate being targeted by a crRNA (top). The protospacer region is highlighted in blue, and the orange bars indicate the 5′ PFS and 3′ PFS sequences. The orange letters represent the altered sequences in the experiment. Denaturing gel showing crRNA-guided ssRNA cleavage activity after 60 minutes of incubation, with the 5′ PFS tested as A, and the 3′ PFS tested as ANN (bottom). The orange 3′ PFS letters represent the RNA bases at the second and third 3′ PFS position within each target ssRNA. Gel lane containing RNA ladder not shown. Dashed line indicates two separate gels shown side by side. (D) Schematic showing the secondary structure of the body labeled ssRNA targets used in the denaturing gel. The variable loop of the schematic (represented as N5) is substituted with five monomers of the variable loop base in the gel (top). Denaturing gel showing cleavage bands of the homopolymer variable loop base (bottom). The targets were incubated for 30 minutes. Dashed line indicates where the image was stitched together to remove U/A heteropolymer RNA lanes (shown in Figure S7B). Gel lane containing RNA ladder not shown. (E) Denaturing gel showing BzCas13b collateral cleavage activity after 30 minutes of incubation, with schematic of cleavage experiment to the right. Two crRNAs (A and B) target substrate 1 (1A and 1B) or substrate 2 (2A and 2B). Gel lane containing RNA ladder not shown. Dashed line indicates two separate gels shown side by side. See also Figures S4 and S6, and Table S1.
Figure 5. HEPN domains mediate RNA cleavage by Cas13b, whose activity is repressed by Csx27
(A) Protospacer design for MS2 phage plaque drop assay to test RNA interference (left). Plaque drop assay for full B. zoohelcum VI-B1 locus (center) and bzcas13b (right). (B) DNA interference assay schematic (top) and results (bottom). A target sequence is placed in frame at the start of the transcribed bla gene that confers ampicillin resistance or in a non-transcribed region on the opposite strand of the same target plasmid. Target plasmids were co-transformed with bzcas13b plasmid or empty vectors conferring chloramphenicol resistance and plated on double selection antibiotic plates. (C) Schematic (top) and denaturing gel (bottom) showing ssRNA cleavage activity of WT and HEPN mutant BzCas13b. The protein and targeting crRNA complexes were incubated for 10 minutes. Gel lane containing RNA ladder not shown. (D) Electrophoretic Mobility Shift Assay (EMSA) graph showing the affinity of BzCas13b proteins and targeting crRNA complex to a 5′ end labeled ssRNA. The EMSA assay was performed with supplemental EDTA to reduce any cleavage activity. (E) Quantification of MS2 phage plaque drop assay with B. zoohelcum wildtype and Q (R116A/H121A/R1177A/H1182A) mutant Cas13b. See also Figures S4, S5, and S6, and Tables S1, S2, S6, and S7.
Figure 6. Efficient RNA targeting by Cas13b is correlated with local RNA accessibility
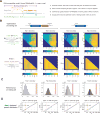
(A) Methodology of secondary structure-mediated spacer efficiency analysis of E. coli essential gene screen data with Vienna RNAplfold. (B) Optimization of top 1 accuracy (computationally predicted most accessible spacer matches the top experimentally depleted spacer) and top 3 accuracy (computationally predicted top spacer falls in top 3 experimentally depleted spacers) on randomly selected B. zoohelcum training dataset using RNAplfold, first with u start and u end, and then with W and L. (C) Performance of optimized RNAplfold model on randomly selected B. zoohelcum testing dataset (48 cohorts for full B. zoohelcum VI-B1 locus, 56 cohorts for bzcas13b) against 106 Monte Carlo simulations: empirical _P_-values from left to right of 3e–6, 1e–6, 8.7e–3, 6e–6. (D) Empirical cumulative distribution function of safely depleted B. zoohelcum spacers over all genes from 5′ UTR into gene and from 3′ UTR into gene. Yellow line separates UTR and gene, red line is theoretical cumulative distribution function of uniformly distributed spacers, and blue line is empirical cumulative distribution of safely depleted B. zoohelcum spacers. See also Tables S3 and S4.
Figure 7. Class 2 type VI-B systems are differentially regulated across two loci by Csx27 and Csx28
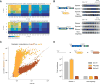
(A) Normalized PFS matrix, for P. buccae VI-B2 locus (left) and pbcas13b (right). (B) MS2 Plaque drop assay for full P. buccae VI-B2 locus (left) and pbcas13b (right). (C) Spacer depletions of bzcas13b with and without bzcsx27 (brown), as compared to pbcas13b with and without pbcsx28 (gold). (D) Fold resistance to MS2 infection for cells co-transformed with pbcas13b and the indicated csx expression plasmid. See also Figures S5 and S7, and Tables S2, S3, S4, and S6.
Similar articles
- Cas13d Is a Compact RNA-Targeting Type VI CRISPR Effector Positively Modulated by a WYL-Domain-Containing Accessory Protein.
Yan WX, Chong S, Zhang H, Makarova KS, Koonin EV, Cheng DR, Scott DA. Yan WX, et al. Mol Cell. 2018 Apr 19;70(2):327-339.e5. doi: 10.1016/j.molcel.2018.02.028. Epub 2018 Mar 15. Mol Cell. 2018. PMID: 29551514 Free PMC article. - Structural Basis for Guide RNA Processing and Seed-Dependent DNA Targeting by CRISPR-Cas12a.
Swarts DC, van der Oost J, Jinek M. Swarts DC, et al. Mol Cell. 2017 Apr 20;66(2):221-233.e4. doi: 10.1016/j.molcel.2017.03.016. Mol Cell. 2017. PMID: 28431230 Free PMC article. - Expanding the CRISPR Toolbox: Targeting RNA with Cas13b.
Barrangou R, Gersbach CA. Barrangou R, et al. Mol Cell. 2017 Feb 16;65(4):582-584. doi: 10.1016/j.molcel.2017.02.002. Mol Cell. 2017. PMID: 28212745 - Molecular Mechanisms of RNA Targeting by Cas13-containing Type VI CRISPR-Cas Systems.
O'Connell MR. O'Connell MR. J Mol Biol. 2019 Jan 4;431(1):66-87. doi: 10.1016/j.jmb.2018.06.029. Epub 2018 Jun 22. J Mol Biol. 2019. PMID: 29940185 Review. - Structures, mechanisms and applications of RNA-centric CRISPR-Cas13.
Yang H, Patel DJ. Yang H, et al. Nat Chem Biol. 2024 Jun;20(6):673-688. doi: 10.1038/s41589-024-01593-6. Epub 2024 May 3. Nat Chem Biol. 2024. PMID: 38702571 Free PMC article. Review.
Cited by
- A CRISPR/RfxCas13d-mediated strategy for efficient RNA knockdown in mouse embryonic development.
Zhang L, Cao SM, Wu H, Yan M, Li J, Chen LL. Zhang L, et al. Sci China Life Sci. 2024 Nov;67(11):2297-2306. doi: 10.1007/s11427-023-2572-6. Epub 2024 Aug 2. Sci China Life Sci. 2024. PMID: 39110403 - CRISPR/Cas13d-Mediated Microbial RNA Knockdown.
Zhang K, Zhang Z, Kang J, Chen J, Liu J, Gao N, Fan L, Zheng P, Wang Y, Sun J. Zhang K, et al. Front Bioeng Biotechnol. 2020 Jul 30;8:856. doi: 10.3389/fbioe.2020.00856. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32850723 Free PMC article. - A Review on the Mechanism and Applications of CRISPR/Cas9/Cas12/Cas13/Cas14 Proteins Utilized for Genome Engineering.
Hillary VE, Ceasar SA. Hillary VE, et al. Mol Biotechnol. 2023 Mar;65(3):311-325. doi: 10.1007/s12033-022-00567-0. Epub 2022 Sep 27. Mol Biotechnol. 2023. PMID: 36163606 Free PMC article. Review. - The Bibliometric Landscape of Gene Editing Innovation and Regulation in the Worldwide.
Wei X, Pu A, Liu Q, Hou Q, Zhang Y, An X, Long Y, Jiang Y, Dong Z, Wu S, Wan X. Wei X, et al. Cells. 2022 Aug 29;11(17):2682. doi: 10.3390/cells11172682. Cells. 2022. PMID: 36078090 Free PMC article. - Powerful CRISPR-Based Biosensing Techniques and Their Integration With Microfluidic Platforms.
Chen B, Li Y, Xu F, Yang X. Chen B, et al. Front Bioeng Biotechnol. 2022 Feb 23;10:851712. doi: 10.3389/fbioe.2022.851712. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35284406 Free PMC article. Review.
References
- Abil Z, Zhao H. Engineering reprogrammable RNA-binding proteins for study and manipulation of the transcriptome. Mol Biosyst. 2015;11:2658–2665. - PubMed
- Bernhart SH, Hofacker IL, Stadler PF. Local RNA base pairing probabilities in large sequences. Bioinformatics. 2006;22:614–615. - PubMed
MeSH terms
Substances
Grants and funding
- DP1 MH100706/MH/NIMH NIH HHS/United States
- R01 MH110049/MH/NIMH NIH HHS/United States
- RM1 HG006193/HG/NHGRI NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials