Topical capsaicin (high concentration) for chronic neuropathic pain in adults - PubMed (original) (raw)
Review
Topical capsaicin (high concentration) for chronic neuropathic pain in adults
Sheena Derry et al. Cochrane Database Syst Rev. 2017.
Abstract
Background: This review is an update of 'Topical capsaicin (high concentration) for chronic neuropathic pain in adults' last updated in Issue 2, 2013. Topical creams with capsaicin are used to treat peripheral neuropathic pain. Following application to the skin, capsaicin causes enhanced sensitivity, followed by a period with reduced sensitivity and, after repeated applications, persistent desensitisation. High-concentration (8%) capsaicin patches were developed to increase the amount of capsaicin delivered; rapid delivery was thought to improve tolerability because cutaneous nociceptors are 'defunctionalised' quickly. The single application avoids noncompliance. Only the 8% patch formulation of capsaicin is available, with a capsaicin concentration about 100 times greater than conventional creams. High-concentration topical capsaicin is given as a single patch application to the affected part. It must be applied under highly controlled conditions, often following local anaesthetic, due to the initial intense burning sensation it causes. The benefits are expected to last for about 12 weeks, when another application might be made.
Objectives: To review the evidence from controlled trials on the efficacy and tolerability of topically applied, high-concentration (8%) capsaicin in chronic neuropathic pain in adults.
Search methods: For this update, we searched CENTRAL, MEDLINE, Embase, two clinical trials registries, and a pharmaceutical company's website to 10 June 2016.
Selection criteria: Randomised, double-blind, placebo-controlled studies of at least 6 weeks' duration, using high-concentration (5% or more) topical capsaicin to treat neuropathic pain.
Data collection and analysis: Two review authors independently searched for studies, extracted efficacy and adverse event data, and examined issues of study quality and potential bias. Where pooled analysis was possible, we used dichotomous data to calculate risk ratio and numbers needed to treat for one additional event, using standard methods.Efficacy outcomes reflecting long-duration pain relief after a single drug application were from the Patient Global Impression of Change (PGIC) at specific points, usually 8 and 12 weeks. We also assessed average pain scores over weeks 2 to 8 and 2 to 12 and the number of participants with pain intensity reduction of at least 30% or at least 50% over baseline, and information on adverse events and withdrawals.We assessed the quality of the evidence using GRADE and created a 'Summary of findings' table.
Main results: We included eight studies, involving 2488 participants, two more studies and 415 more participants than the previous version of this review. Studies were of generally good methodological quality; we judged only one study at high risk of bias, due to small size. Two studies used a placebo control and six used 0.04% topical capsaicin as an 'active' placebo to help maintain blinding. Efficacy outcomes were inconsistently reported, resulting in analyses for most outcomes being based on less than complete data.For postherpetic neuralgia, we found four studies (1272 participants). At both 8 and 12 weeks about 10% more participants reported themselves much or very much improved with high-concentration capsaicin than with 'active' placebo, with point estimates of numbers needed to treat for an additional beneficial outcome (NNTs) of 8.8 (95% confidence interval (CI) 5.3 to 26) with high-concentration capsaicin and 7.0 (95% CI 4.6 to 15) with 'active' placebo (2 studies, 571 participants; moderate quality evidence). More participants (about 10%) had average 2 to 8-week and 2 to 12-week pain intensity reductions over baseline of at least 30% and at least 50% with capsaicin than control, with NNT values between 10 and 12 (2 to 4 studies, 571 to 1272 participants; very low quality evidence).For painful HIV-neuropathy, we found two studies (801 participants). One study reported the proportion of participants who were much or very much improved at 12 weeks (27% with high-concentration capsaicin and 10% with 'active' placebo). For both studies, more participants (about 10%) had average 2 to 12-week pain intensity reductions over baseline of at least 30% with capsaicin than control, with an NNT of 11 (very low quality evidence).For peripheral diabetic neuropathy, we found one study (369 participants). It reported about 10% more participants who were much or very much improved at 8 and 12 weeks. One small study of 46 participants with persistent pain following inguinal herniorrhaphy did not show a difference between capsaicin and placebo for pain reduction (very low quality evidence).We downgraded the quality of the evidence for efficacy outcomes by one to three levels due to sparse data, imprecision, possible effects of imputation methods, and susceptibility to publication bias.Local adverse events were common, but not consistently reported. Serious adverse events were no more common with active treatment (3.5%) than control (3.2%). Adverse event withdrawals did not differ between groups, but lack of efficacy withdrawals were somewhat more common with control than active treatment, based on small numbers of events (six to eight studies, 21 to 67 events; moderate quality evidence, downgraded due to few events). No deaths were judged to be related to study medication.
Authors' conclusions: High-concentration topical capsaicin used to treat postherpetic neuralgia, HIV-neuropathy, and painful diabetic neuropathy generated more participants with moderate or substantial levels of pain relief than control treatment using a much lower concentration of capsaicin. These results should be interpreted with caution as the quality of the evidence was moderate or very low. The additional proportion who benefited over control was not large, but for those who did obtain high levels of pain relief, there were usually additional improvements in sleep, fatigue, depression, and quality of life. High-concentration topical capsaicin is similar in its effects to other therapies for chronic pain.
Conflict of interest statement
SD: none known.
ASCR undertakes consultancy and advisory board work for Imperial College Consultants ‐ since June 2013 this has included remunerated work for: Spinifex, Abide, Astellas, Neusentis, Merck, Medivir, Mitsubishi, Aquilas, Asahi Kasei, Relmada, Novartis, and Orion. All consultancy activity relates to consultancy advice on the preclinical/clinical development of drugs for neuropathic pain. Neusentis was a subsidiary of Pfizer. He owned share options in Spinifex Pharmaceuticals which was acquired by Novartis in July 2015. ASCR was a Principal Investigator in the EuroPain consortium. EuroPain has received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement number 115007, resources for which are composed of financial contribution from the European Union's Seventh Framework Programme (FP7/20072013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies (www.imieuropain.org). Specifically, research funding for ASCR's laboratory has been received by Imperial College from Pfizer (manufacturer of gabapentin) and Astellas ‐ both these grants were for projects related to improving the validity of animal models of neuropathic pain. ASCR is a site investigator for the Neuropain project, funded by Pfizer via Kiel University ‐ Chief Investigator Prof Ralf Baron. He is Vice‐Chair of the International Association for the Study of Pain (IASP) Special Interest Group on Neuropathic Pain (www.neupsig.org) and serves on the Executive Committee of ACTTION (Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks; www.acttion.org).
PC received support from Boston Scientific (2014) for travel and accommodation at a scientific meeting; Boston Scientific does not market drugs. PC is a specialist pain physician and manages patients with chronic pain.
TT: none known.
RAM has received grant support from Grünenthal relating to individual patient level analyses of trial data regarding tapentadol in osteoarthritis and back pain (2015). He has received honoraria for attending boards with Menarini concerning methods of analgesic trial design (2014), with Novartis (2014) about the design of network meta‐analyses, and RB on understanding pharmacokinetics of drug uptake (2015). He has received honoraria from Omega Pharma (2016) and Futura Pharma (2016) for providing advice on trial and data analysis methods.
This review was identified in a 2019 audit as not meeting the current definition of the Cochrane Commercial Sponsorship policy. At the time of its publication it was compliant with the interpretation of the existing policy. As with all reviews, new and updated, at update this review will be revised according to 2020 policy update.
Figures
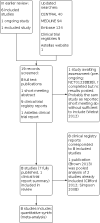
1
Study flow diagram.
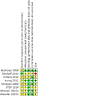
2
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
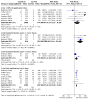
3
Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.1 Postherpetic neuralgia ‐ at least 50% pain intensity reduction over weeks 2 to 8.
4
Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.5 Postherpetic neuralgia ‐ Patient Global Impression of Change much or very much improved at 8 and 12 weeks.
5
Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.6 HIV‐neuropathy ‐ at least 30% pain intensity reduction over weeks 2 to 12.
6
Forest plot of comparison: 1 High‐concentration (8%) capsaicin versus control (single dose), outcome: 1.10 Serious adverse events.
1.1. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 1: Postherpetic neuralgia (PHN) ‐ at least 50% pain intensity reduction over weeks 2 to 8
1.2. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 2: PHN ‐ at least 50% pain intensity reduction over 2 to 12 weeks
1.3. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 3: PHN ‐ at least 30% pain intensity reduction over weeks 2 to 8
1.4. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 4: PHN ‐ at least 30% pain intensity reduction over weeks 2 to 12
1.5. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 5: PHN ‐ Patient Global Impression of ChangePGIC much or very much improved at 8 and 12 weeks
1.6. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 6: HIV‐neuropathy ‐ at least 30% pain intensity reduction over weeks 2 to 12
1.7. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 7: Local skin reactions ‐ group 1
1.8. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 8: Local skin reactions ‐ group 2
1.9. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 9: Patch tolerability
1.10. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 10: Serious adverse events
1.11. Analysis
Comparison 1: High‐concentration (8%) capsaicin versus control (single dose), Outcome 11: Withdrawals
Update of
- Topical capsaicin (high concentration) for chronic neuropathic pain in adults.
Derry S, Sven-Rice A, Cole P, Tan T, Moore RA. Derry S, et al. Cochrane Database Syst Rev. 2013 Feb 28;(2):CD007393. doi: 10.1002/14651858.CD007393.pub3. Cochrane Database Syst Rev. 2013. PMID: 23450576 Updated. Review.
Similar articles
- Topical capsaicin (high concentration) for chronic neuropathic pain in adults.
Derry S, Sven-Rice A, Cole P, Tan T, Moore RA. Derry S, et al. Cochrane Database Syst Rev. 2013 Feb 28;(2):CD007393. doi: 10.1002/14651858.CD007393.pub3. Cochrane Database Syst Rev. 2013. PMID: 23450576 Updated. Review. - Gabapentin for chronic neuropathic pain in adults.
Wiffen PJ, Derry S, Bell RF, Rice AS, Tölle TR, Phillips T, Moore RA. Wiffen PJ, et al. Cochrane Database Syst Rev. 2017 Jun 9;6(6):CD007938. doi: 10.1002/14651858.CD007938.pub4. Cochrane Database Syst Rev. 2017. PMID: 28597471 Free PMC article. Review. - Oxycodone for neuropathic pain and fibromyalgia in adults.
Gaskell H, Moore RA, Derry S, Stannard C. Gaskell H, et al. Cochrane Database Syst Rev. 2014 Jun 23;(6):CD010692. doi: 10.1002/14651858.CD010692.pub2. Cochrane Database Syst Rev. 2014. PMID: 24956205 Updated. Review. - Tramadol for neuropathic pain in adults.
Duehmke RM, Derry S, Wiffen PJ, Bell RF, Aldington D, Moore RA. Duehmke RM, et al. Cochrane Database Syst Rev. 2017 Jun 15;6(6):CD003726. doi: 10.1002/14651858.CD003726.pub4. Cochrane Database Syst Rev. 2017. PMID: 28616956 Free PMC article. Review. - Gabapentin for chronic neuropathic pain and fibromyalgia in adults.
Moore RA, Wiffen PJ, Derry S, Toelle T, Rice AS. Moore RA, et al. Cochrane Database Syst Rev. 2014 Apr 27;2014(4):CD007938. doi: 10.1002/14651858.CD007938.pub3. Cochrane Database Syst Rev. 2014. PMID: 24771480 Free PMC article. Updated. Review.
Cited by
- Antinociceptive Activity of Chemical Components of Essential Oils That Involves Docking Studies: A Review.
Assis DB, Aragão Neto HC, da Fonsêca DV, de Andrade HHN, Braga RM, Badr N, Maia MDS, Castro RD, Scotti L, Scotti MT, de Almeida RN. Assis DB, et al. Front Pharmacol. 2020 May 29;11:777. doi: 10.3389/fphar.2020.00777. eCollection 2020. Front Pharmacol. 2020. PMID: 32547391 Free PMC article. - Therapeutic Approaches for Peripheral and Central Neuropathic Pain.
Szok D, Tajti J, Nyári A, Vécsei L. Szok D, et al. Behav Neurol. 2019 Nov 21;2019:8685954. doi: 10.1155/2019/8685954. eCollection 2019. Behav Neurol. 2019. PMID: 31871494 Free PMC article. Review. - Evaluation of 8% Capsaicin Patches in Chemotherapy-Induced Peripheral Neuropathy: A Retrospective Study in a Comprehensive Cancer Center.
Bienfait F, Julienne A, Jubier-Hamon S, Seegers V, Delorme T, Jaoul V, Pluchon YM, Lebrec N, Dupoiron D. Bienfait F, et al. Cancers (Basel). 2023 Jan 5;15(2):349. doi: 10.3390/cancers15020349. Cancers (Basel). 2023. PMID: 36672298 Free PMC article. - Pregabalin for neuropathic pain in adults.
Derry S, Bell RF, Straube S, Wiffen PJ, Aldington D, Moore RA. Derry S, et al. Cochrane Database Syst Rev. 2019 Jan 23;1(1):CD007076. doi: 10.1002/14651858.CD007076.pub3. Cochrane Database Syst Rev. 2019. PMID: 30673120 Free PMC article. - Postherpetic Neuralgia: Current Evidence on the Topical Film-Forming Spray with Bupivacaine Hydrochloride and a Review of Available Treatment Strategies.
Ngo AL, Urits I, Yilmaz M, Fortier L, Anya A, Oh JH, Berger AA, Kassem H, Sanchez MG, Kaye AD, Urman RD, Herron EW, Cornett EM, Viswanath O. Ngo AL, et al. Adv Ther. 2020 May;37(5):2003-2016. doi: 10.1007/s12325-020-01335-9. Epub 2020 Apr 15. Adv Ther. 2020. PMID: 32297285 Free PMC article. Review.
References
References to studies included in this review
Backonja 2008 {published data only}
- Backonja M, Wallace MS, Blonsky ER, Cutler BJ, Malan P Jr, Rauck R, et al, NGX-4010 C107 Study Group. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomised, double-blind study. Lancet Neurology 2008;7(12):1106-12. [DOI: 10.1016/S1474-4422(08)70228-X] - DOI - PubMed
Bischoff 2014 {published data only}
- Bischoff JM, Ringsted TK, Petersen M, Sommer C, Uçeyler N, Werner MU. A capsaicin (8%) patch in the treatment of severe persistent inguinal postherniorrhaphy pain: a randomized, double-blind, placebo-controlled trial. PLoS One 2014;9(10):e109144. [DOI: 10.1371/journal.pone.0109144] - DOI - PMC - PubMed
Clifford 2012 {published data only}
- Brown S, Simpson DM, Moyle G, Brew BJ, Schifitto G, Larbalestier N, et al. NGX-4010, a capsaicin 8% patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: integrated analysis of two phase III, randomized, controlled trials. AIDS Research and Therapy 2013;10(1):5. [DOI: 10.1186/1742-6405-10-5] - DOI - PMC - PubMed
- Clifford DB, Simpson DM, Brown S, Moyle G, Brew BJ, Conway B, et al, NGX-4010 C119 Study Group. A randomized, double-blind, controlled study of NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy. Journal of Acquired Immune Deficiency Syndromes 2012;589(2):126-33. [DOI: 10.1097/QAI.0b013e31823e31f7] - DOI - PubMed
Irving 2011 {published data only}
- Irving GA, Backonja MM, Dunteman E, Blonsky ER, Vanhove GF, Lu SP, et al, NGX-4010 C117 Study Group. A multicenter, randomized, double-blind, controlled study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Pain Medicine 2011;12(1):99-109. [DOI: 10.1111/j.1526-4637.2010.01004.x] - DOI - PubMed
Simpson 2008 {published data only}
- Brown S, Simpson DM, Moyle G, Brew BJ, Schifitto G, Larbalestier N, et al. NGX-4010, a capsaicin 8% patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: integrated analysis of two phase III, randomized, controlled trials. AIDS Research and Therapy 2013;10(1):5. [DOI: 10.1186/1742-6405-10-5] - DOI - PMC - PubMed
STEP 2014 {unpublished data only}
- Astellas PharmaEurope BV (Sponsor). A phase III, double-blind, randomized, placebo-controlled, multicenter study evaluating the efficacy and safety of QUTENZA® in subjects with painful diabetic peripheral neuropathy (clinical study results). www.astellasclinicalstudyresults.com/hcp/study.aspx?ID=76 Date first received: 6 February 2012. [ASTELLAS ID: E05-CL-3004]
- Astellas Pharma Inc (Responsible party). A phase III, double-blind, randomized, placebo-controlled, multicenter study evaluating the efficacy and safety of QUTENZA® in subjects with painful diabetic peripheral neuropathy. clinicaltrials.gov/ct2/show/NCT01533428 Date first received: 12 February 2012. [ASTELLAS ID: E05-CL-3004] [CTG: ]
Webster 2010a {published data only}
- Webster LR, Tark M, Rauck R, Tobias JK, Vanhove GF. Effect of duration of postherpetic neuralgia on efficacy analyses in a multicenter, randomized, controlled study of NGX-4010, an 8% capsaicin patch evaluated for the treatment of postherpetic neuralgia. BMC Neurology 2010;10:92. [DOI: 10.1186/1471-2377-10-92] - DOI - PMC - PubMed
Webster 2010b {published data only}
- Webster LR, Malan TP, Tuchman MM, Mollen MD, Tobias JK, Vanhove GF. A multicenter, randomized, double-blind, controlled dose finding study of NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia. Journal of Pain 2010;11(10):972-82. [DOI: 10.1016/j.jpain.2010.01.270] - DOI - PubMed
References to studies excluded from this review
Backonja 2010 {published data only}
- Backonja MM, Malan TP, Vanhove GF, Tobias JK, C102/106 Study Group. NGX-4010, a high-concentration capsaicin patch, for the treatment of postherpetic neuralgia: a randomized, double-blind, controlled study with an open-label extension. Pain Medicine 2010;11(4):600-8. [DOI: 10.1111/j.1526-4637.2009.00793.x] - DOI - PubMed
References to studies awaiting assessment
NCT01228838 {published data only}
- Vanhove T. A multicenter randomized, double-blind, controlled study to evaluate safety, tolerability and preliminary efficacy of two capsaicin concentration variations of NGX-1998 (10% or 20% w/w) in subjects with postherpetic neuralgia (PHN). clinicaltrials.gov/ct2/show/NCT01228838 Date first received: 25 October 2010. [CTG: ] [NEUROGESX ID: C204]
- Webster L, Bhattacharya S, Wallace M, Wells B, Tobias J, Babbar S. Efficacy and safety of NGX-1998, a novel topical liquid formulation of capsaicin, in patients with postherpetic neuralgia: results of a multi-center, placebo-controlled trial. Journal of Pain 2012;13 (4 Suppl 1):S72. [DOI: 10.1016/j.jpain.2012.01.300] - DOI
Additional references
Anand 2011
Azevedo 2016
Baron 2012
Bouhassira 2008
Bouhassira 2012
Burness 2016
Calvo 2012
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain 1997;72(1-2):95-7. - PubMed
Cook 1995
Dechartres 2013
Demant 2014
- Demant DT, Lund K, Vollert J, Maier C, Segerdahl M, Finnerup NB, et al. The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 2014;155(11):2263-73. [DOI: 10.1016/j.pain.2014.08.014] - DOI - PubMed
Derry 2012
Derry 2014
Dworkin 2008
Edwards 1999
ELEVATE 2014
- Astellas PharmaEurope BV (Sponsor). QUTENZATM versus pregabalin in subjects with peripheral neuropathic pain: an open-label, randomized, multicenter, non-inferiority efficacy and tolerability study. www.astellasclinicalstudyresults.com/hcp/study.aspx?ID=83 Date first received: 11 July 2012. [EUDRACT NUMBER: 2011-005872-41]
eMC 2012
- eMC. Qutenza 179mg cutaneous patch. www.medicines.org.uk/emc/medicine/23156/SPC/qutenza 179mg cutaneous patch/ (accessed 28 April 2012).
EPOC 2015
- Effective Practice and Organisation of Care (EPOC). 23. Worksheets for preparing a Summary of Findings using GRADE. Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available at: epoc.cochrane.org/epoc-specific-resources-review-authors (accessed 30 November 2016) 2015.
FDA 1999
- US Department of Health and Human Services. Guidance for industry: skin irritation and sensitization testing of generic transdermal drug products. www.fda.gov/ohrms/dockets/98fr/990236Gd.pdf (accessed 13 December 2016).
Finnerup 2013
Finnerup 2015
Gülfe 2010
- Gülfe A, Kristensen LE, Saxne T, Jacobsson LT, Petersson IF, Geborek P. Utility-based outcomes made easy: the number needed per quality-adjusted life year gained. An observational cohort study of tumor necrosis factor blockade in inflammatory arthritis from Southern Sweden. Arthritis Care and Research 2010;62(10):1399-406. [DOI: 10.1002/acr.20235] - DOI - PubMed
Gustorff 2008
- Gustorff B, Dorner T, Likar R, Grisold W, Lawrence K, Schwarz F, et al. Prevalence of self-reported neuropathic pain and impact on quality of life: a prospective representative survey. Acta Anaesthesiological Scandinavica 2008;52(1):132-6. - PubMed
Guyatt 2011
Guyatt 2013a
- Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. Journal of Clinical Epidemiology 2013;66(2):151-7. [DOI: 10.1016/j.jclinepi.2012.01.006] - DOI - PubMed
Guyatt 2013b
Hall 2008
Hall 2013
Helfert 2015
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hoffman 2010
Ikenberg 2012
- Ikenberg R, Hertel N, Moore RA, Obradovic M, Baxter G, Conway P, et al. Cost-effectiveness of tapentadol prolonged release compared with oxycodone controlled release in the UK in patients with severe non-malignant chronic pain who failed 1st line treatment with morphine. Journal of Medical Economics 2012;15(4):724-36. [DOI: 10.3111/13696998.2012.670174] - DOI - PubMed
Jadad 1996
Jensen 2011
Kalso 2013
Katusic 1991
Kern 2014
Knolle 2013
Koopman 2009
L'Abbé 1987
Loke 2001
Lunn 2014
Moore 1998
Moore 2005
- Moore RA, Derry S, Makinson GT, McQuay HJ. Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports. Arthritis Research and Therapy 2005;7(3):R644-65. [DOI: 10.1186/ar1704] - DOI - PMC - PubMed
Moore 2008a
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15-23. [ISBN: 978-0-931092-69-5]
Moore 2008b
Moore 2009
Moore 2011a
Moore 2011b
Moore 2012a
Moore 2012b
Moore 2013a
Moore 2013b
Moore 2014a
Moore 2014b
Moore 2014c
Morris 1995
Mou 2013
NICE 2013
- National Institute for Health and Care Excellence (NICE). Neuropathic pain - pharmacological management: the pharmacological management of neuropathic pain in adults in non-specialist settings, 2013. www.nice.org.uk/guidance/cg173 (accessed 30 November 2016).
Nüesch 2010
O'Brien 2010
PACE 2014
- Astellas PharmaEurope BV (Sponsor). A randomized, controlled, long-term safety study evaluating the effect of repeated applications of QUTENZATM plus standard of care versus standard of care alone in patients with painful diabetic peripheral neuropathy. www.astellasclinicalstudyresults.com/hcp/study.aspx?ID=75 Date first received: 10 November 2011. [ASTELLAS ID: E05-CL-3002] [CTG: ] [EUDRACT NUMBER: 2009-016458-42]
PaPaS 2012
- PaPaS author and referee guidance. papas.cochrane.org/papas-documents (accessed 30 November 2016).
Phillips 2010
Rappaport 1994
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Simpson 2014
- Simpson DM, Brown S, Tobias JK, Vanhove GF, NGX-4010 C107 Study Group. NGX-4010, a capsaicin 8% dermal patch, for the treatment of painful HIV-associated distal sensory polyneuropathy: results of a 52-week open-label study. Clinical Journal of Pain 2014;30(2):134-42. [DOI: 10.1097/AJP.0b013e318287a32f] - DOI - PubMed
Straube 2010
Straube 2011
STRIDE 2014
- Astellas PharmaEurope BV (Sponsor). A multicentre, single-arm, open-label study of the repeated administration of QUTENZATM for the treatment of peripheral neuropathic pain. www.astellasclinicalstudyresults.com/hcp/study.aspx?ID=81 Date first received: 28 October 2010. [EUDRACT NUMBER: 2009-016457-18]
Sultan 2008
Torrance 2006
Treede 2008
Űçeyler 2014
van Hecke 2014
van Nooten 2015
- Nooten FE, Charokopou M, Poole C, Treur M. A systematic literature review and network meta-analysis of capsaicin 8% patch versus oral neuropathic pain medications for the treatment of painful diabetic peripheral neuropathy. Value in Health 2015;18(7):A659. [DOI: 10.1016/j.jval.2015.09.2388] - DOI - PubMed
von Hehn 2012
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163-96. [DOI: 10.1016/S0140-6736(12)61729-2] - DOI - PMC - PubMed
Wiffen 2013
References to other published versions of this review
Derry 2009
Derry 2013
Mason 2004
- Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ (Clinical Research Ed) 2004;328(7446):991. [10.1136/bmj.38042.506748.EE] - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical