The readily releasable pool of synaptic vesicles - PubMed (original) (raw)
Review
The readily releasable pool of synaptic vesicles
Pascal S Kaeser et al. Curr Opin Neurobiol. 2017 Apr.
Abstract
Each presynaptic bouton is densely packed with many vesicles, only a small fraction of which are available for immediate release. These vesicles constitute the readily releasable pool (RRP). The RRP size, and the probability of release of each vesicle within the RRP, together determine synaptic strength. Here, we discuss complications and recent advances in determining the size of the physiologically relevant RRP. We consider molecular mechanisms to generate and regulate the RRP, and discuss the relationship between vesicle docking and the RRP. We conclude that many RRP vesicles are docked, that some docked vesicles may not be part of the RRP, and that undocked vesicles can contribute to the RRP by rapid recruitment to unoccupied, molecularly activated ready-to-release sites.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
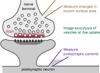
Figure 1. Measurements of RRP
A schematic of a synapse is shown with a presynaptic nerve terminal containing many vesicles. Some of these vesicles are close to the active zone and make up the RRP. To quantify the RRP size it is necessary to quantify neurotransmitter release, which is done in several different ways. It is possible to record directly from some types of presynaptic boutons (top), and this allows control of the presynaptic potential for large voltage steps, allows control of the intracellular milieu, and makes it possible to measure the change in surface area in response to vesicle fusion. It is also possible to quantify fusion using optical methods (middle, illustrated by vesicles colored in green). The most common method to quantify RRP size is to record postsynaptic currents (bottom).
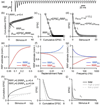
Figure 2. Using synaptic responses evoked by high-frequency stimulus trains to estimate synaptic parameters
Synaptic responses are described by N0 (the size of the readily releasable pool, RRP), p (the vesicular release probability), R (the rate of replenishment of the RRP from a reserve pool) and q (the size of a quantal response). (a). Simulated EPSCs in response to a 100 Hz stimulus train. (b, c). Two extrapolation methods commonly used to estimate synaptic parameters are illustrated: one referred to as the train method (b) and the other as the Elmqvist and Quastel (EQ) method. (d). If depression of synaptic responses is due to RRP depletion, the dependence of the EPSC amplitude on number of stimuli can be used to estimate p and determine the RRP (from [8*]). (e–f) Simulations based on a depletion model were used to determine EPSC amplitudes during a train and the cumulative train method and EQ methods were used to estimate the RRP from these simulated responses (from [8*]). The dashed line corresponds to the RRP size used in the simulations. (h–i) Simulations with a depletion model were made for a synapse with 50% of release having p=0.4 and 50% having p=0.04. Plots were made as in B–D that highlight complications associated with having nonuniform p.
Figure 3. Simplified, Munc13-centered model of exocytosis
Munc13 participates in multiple steps of exocytosis, which raises the question at which step a vesicle becomes part of the RRP. (a) RIM recruits and monomerizes Munc13 to activate a release site. (b) Munc13, together with Munc18, opens syntaxin-1 to allow for the assembly of the SNARE complex. (c) SNARE complexes may partially assemble under the molecular control of Munc13 and Munc18, and this assembly may be regulated by complexin, synaptotagmin, or other SNARE-binding proteins. (d) Fusion proceeds when SNARE proteins fully assemble into a four-alpha-helical bundle that forces the vesicular and target membranes to fuse.
Figure 4. Morphological correlates of RRP
The RRP consits of docked vesicles. The questions that arise are: Are all RRP vesicles docked? Are all docked vesicles in the RRP? (a) One model posits that all docked vesicles are part of the RRP and all RRP vesicles are docked. (b) Another possiblity is that only a subset of docked vesicles is the RRP. (c) A third model is that many RRP vesicles are docked, but additional vesicles may contribute to RRP through rapid recruitment to empty, activated release sites. In (a) – (c), RRP vesicles are illustrated in red and the active zone is the grey shaded area.
Similar articles
- Release probability is regulated by the size of the readily releasable vesicle pool at excitatory synapses in hippocampus.
Dobrunz LE. Dobrunz LE. Int J Dev Neurosci. 2002 Jun-Aug;20(3-5):225-36. doi: 10.1016/s0736-5748(02)00015-1. Int J Dev Neurosci. 2002. PMID: 12175858 - [Presynaptic mechanisms of learning and memory].
Yawo H, Ishizuka T. Yawo H, et al. Brain Nerve. 2008 Jul;60(7):725-36. Brain Nerve. 2008. PMID: 18646612 Review. Japanese. - Three small vesicular pools in sequence govern synaptic response dynamics during action potential trains.
Tran V, Miki T, Marty A. Tran V, et al. Proc Natl Acad Sci U S A. 2022 Feb 1;119(5):e2114469119. doi: 10.1073/pnas.2114469119. Proc Natl Acad Sci U S A. 2022. PMID: 35101920 Free PMC article. - Release Mode Dynamically Regulates the RRP Refilling Mechanism at Individual Hippocampal Synapses.
Kim Y, Lee U, Choi C, Chang S. Kim Y, et al. J Neurosci. 2020 Oct 28;40(44):8426-8437. doi: 10.1523/JNEUROSCI.3029-19.2020. Epub 2020 Sep 28. J Neurosci. 2020. PMID: 32989096 Free PMC article. - Merits and Limitations of Vesicle Pool Models in View of Heterogeneous Populations of Synaptic Vesicles.
Neher E. Neher E. Neuron. 2015 Sep 23;87(6):1131-1142. doi: 10.1016/j.neuron.2015.08.038. Neuron. 2015. PMID: 26402599 Review.
Cited by
- Non-canonical function of ADAM10 in presynaptic plasticity.
Bär J, Fanutza T, Reimann CC, Seipold L, Grohe M, Bolter JR, Delfs F, Bucher M, Gee CE, Schweizer M, Saftig P, Mikhaylova M. Bär J, et al. Cell Mol Life Sci. 2024 Aug 9;81(1):342. doi: 10.1007/s00018-024-05327-8. Cell Mol Life Sci. 2024. PMID: 39123091 Free PMC article. - Synaptic vesicle release during ribbon synapse formation of cone photoreceptors.
Davison A, Gierke K, Brandstätter JH, Babai N. Davison A, et al. Front Cell Neurosci. 2022 Nov 4;16:1022419. doi: 10.3389/fncel.2022.1022419. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36406751 Free PMC article. - A theory of synaptic transmission.
Wang B, Dudko OK. Wang B, et al. Elife. 2021 Dec 31;10:e73585. doi: 10.7554/eLife.73585. Elife. 2021. PMID: 34970965 Free PMC article. - Distinct active zone protein machineries mediate Ca2+ channel clustering and vesicle priming at hippocampal synapses.
Emperador-Melero J, Andersen JW, Metzbower SR, Levy AD, Dharmasri PA, de Nola G, Blanpied TA, Kaeser PS. Emperador-Melero J, et al. Nat Neurosci. 2024 Sep;27(9):1680-1694. doi: 10.1038/s41593-024-01720-5. Epub 2024 Aug 19. Nat Neurosci. 2024. PMID: 39160372 Free PMC article. - Fast resupply of synaptic vesicles requires synaptotagmin-3.
Weingarten DJ, Shrestha A, Juda-Nelson K, Kissiwaa SA, Spruston E, Jackman SL. Weingarten DJ, et al. Nature. 2022 Nov;611(7935):320-325. doi: 10.1038/s41586-022-05337-1. Epub 2022 Oct 19. Nature. 2022. PMID: 36261524
References
Publication types
MeSH terms
Grants and funding
- R01 NS032405/NS/NINDS NIH HHS/United States
- R01 NS083898/NS/NINDS NIH HHS/United States
- R35 NS097284/NS/NINDS NIH HHS/United States
- U54 HD090255/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources