BabA dependent binding of Helicobacter pylori to human gastric mucins cause aggregation that inhibits proliferation and is regulated via ArsS - PubMed (original) (raw)
BabA dependent binding of Helicobacter pylori to human gastric mucins cause aggregation that inhibits proliferation and is regulated via ArsS
Emma C Skoog et al. Sci Rep. 2017.
Abstract
Mucins in the gastric mucus layer carry a range of glycan structures, which vary between individuals, can have antimicrobial effect or act as ligands for Helicobacter pylori. Mucins from various individuals and disease states modulate H. pylori proliferation and adhesin gene expression differently. Here we investigate the relationship between adhesin mediated binding, aggregation, proliferation and adhesin gene expression using human gastric mucins and synthetic adhesin ligand conjugates. By combining measurements of optical density, bacterial metabolic activity and live/dead stains, we could distinguish bacterial aggregation from viability changes, enabling elucidation of mechanisms behind the anti-prolific effects that mucins can have. Binding of H. pylori to Leb-glycoconjugates inhibited the proliferation of the bacteria in a BabA dependent manner, similarly to the effect of mucins carrying Leb. Furthermore, deletion of arsS lead to a decrease in binding to Leb-glycoconjugates and Leb-decorated mucins, accompanied by decreased aggregation and absence of anti-prolific effect of mucins and Leb-glycoconjugates. Inhibition of proliferation caused by adhesin dependent binding to mucins, and the subsequent aggregation suggests a new role of mucins in the host defense against H. pylori. This aggregating trait of mucins may be useful to incorporate into the design of adhesin inhibitors and other disease intervention molecules.
Figures
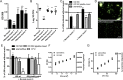
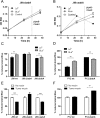
Figure 1. Proliferation and viability in response to different mucins.
(A) OD560 of liquid cultures of H. pylori J99 cultured for 60 h with purified human gastric mucins from several individuals. H. pylori were still in the growth phase at this time point (i.e. had not entered the stationary phase). (B) CFU count of H. pylori J99 cultured for 60 h with human mucins. (C) Comparison between metabolic activity, measured as reduced alamarBlue, and OD560 of H. pylori J99 cultured for 24 h with or without the normal mucin sample 1 (does not induce formation of aggregates, but carries the α1,4-GlcNAc structure that has antibiotic like properties) and the tumor mucin sample (induce formation of aggregates and does not carry α1,4-GlcNAc). Cultures were shaken or pipette-mixed prior to OD560 measurement. Values are shown as percentage of J99 cultured without mucins (represents 100%), n.a. = not analyzed. (D) LIVE/DEAD Bac_Light staining of the J99 wt and J99Δ_babA_Δ_sabA deletion mutant after culture with Leb-positive mucins isolated from a normal stomach (normal mucin 3). Live bacteria are colored green and dead bacteria are colored red. (E) Comparison between OD560 measurements, metabolic activity and CFU counts for J99 wt and J99Δ_babA_Δ_sabA_ deletion mutant after culture with Leb-positive mucin sample 5. Values are shown as percentage of each strain cultured without mucins (represents 100%). (F) The relationship between the alamarBlue signal, CFU counts and OD560 in the absence of mucin. (G) The relationship between the % change in alamarBlue signal at OD560, CFU counts and OD560 in the absence of mucin (100% corresponds to the highest value in (F). All values are presented as mean ± S.E.M, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001: statistical tests were performed with ANOVA with Dunnett’s post hoc test, except the CFU data in panel E, which was analyzed with the Mann-Whitney U test.
Figure 2. Proliferation and adhesion of J99 and P12 in response to Leb- and SLex-glycoconjugates.
(A) Binding of J99 and P12 to Leb- and SLex-glycoconjugates and to the tumor and normal mucin sample analyzed using the microtiter plate based assay (n = 4). (B) Analysis of the metabolic activity as the percentage of reduced alamarBlue in relation to parallel cultures without glycoconjugates (represents 100%) demonstrated a loss of viability in the cultures with Leb-glycoconjugates (n = 6). Values are mean ± S.E.M, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001. ANOVA with Bonferroni’s post hoc test or Student t-test, aap ≤ 0.01, aaap ≤ 0.001 ANOVA with Bonferroni’s post hoc test, compared to proliferation in the absence of glycoconjugates. The results in the graphs have been reproduced at least twice with the same outcome.
Figure 3. Binding of J99 and P12 wt and isogenic adhesin mutants to glycoconjugates and mucins.
Binding of strain J99 (A) and P12 (B) to Leb- and SLex-glycoconjugates, the tumor derived and normal mucin coated onto microtiter plates (n = 4). The results in the graphs have been reproduced at least twice with the same outcome. Values are mean ± S.E.M. Stars indicate statistical relationship of mutant binding compared to the binding of the isogenic wt to the same ligand, One-way ANOVA with Bonferroni’s post hoc test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001, whereas the letter a indicates that the binding (depicted after subtraction of the background signal) is statistically different (p < 0.05) from the background signal. Binding of strain J99 (C) and P12 (D) to Leb- and SLex-glycoconjugates in solution using RadioImmuno assay (n = 9–10). Values are median ± interquartile range. Stars indicate statistical relationship of mutant binding compared to the binding of the isogenic wt to the same ligand, Kruskal-Walllis One-way ANOVA on ranks with Dunn’s multiple comparisons test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
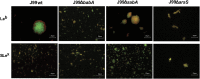
Figure 4. Images of aggregates formed after culturing J99 wt and its isogenic arsS, babA and sabA deletion mutants with glycoconjugates.
Live (green color) and dead (red color) J99 wt, J99Δ_arsS,_ J99Δ_babA and_ J99Δ_sabA_ after 24 h culture with Leb- and SLex-glycoconjugates and stained with the LIVE/DEAD _Bac_Light bacterial viability kit. The images are representative of the whole sample, and of three experiments.
Figure 5. Proliferation of J99 and P12 and isogenic adhesin mutants after culture with glycoconjugates and mucins.
(A,B) OD560 of J99Δ_babA_ and J99Δ_sabA_ during culture in the presence Leb- and SLex-glycoconjugates. Endpoints of cultures were mixed by pipetting to break aggregates. (C,D) Viability of H. pylori after 24 h culture in the presence of the glycoconjugates as measured by alamarBlue reduction. (E,F) Viability of bacteria after 24 h culture in the presence of the tumor mucin sample as measured by alamarBlue reduction. All values are mean ± S.E.M, **p ≤ 0.01, ***p ≤ 0.001, Student’s t-test, n = 6. The results in the graphs have been reproduced at least twice with the same outcome.
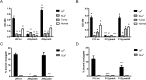
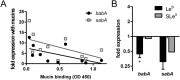
Figure 6. Expression of babA and sabA in response to mucins and ligand binding.
(A) Correlation between mucin binding of J99 wt (as analyzed by microtiter based assay) to 10 different (individual) human mucin samples and gene expression of babA (p = 0.068, r = −0.597) and sabA (p = 0.085, r = −0.571, Pearson correlation) in J99 wt after 24 h culture with these mucins. 9 of the 10 mucins were positive for Leb . (B) Expression of babA and sabA in H. pylori J99 after 24 h culture with Leb- and SLex-glycoconjugates. Stars indicate change of expression compared to untreated bacteria confirmed by paired sample t-test, where Ct values of each experiment were paired to remove confounding factors of variable base levels between experiments. Fold expression compared to bacteria cultured without glycoconjugates or mucins are shown where expression is calculated as ΔCt in relation to expression of 16S rRNA as a housekeeping gene. Values are mean ± S.E.M. from 2 biological replicates, where each data point consists of the mean of 2 technical replicates with very similar result, *p ≤ 0.05.
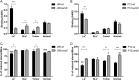
Figure 7. Binding of J99Δ_arsS_ and P12Δ_arsS_ to mucins and glycoconjugates and their viability in response to these mucins and glycoconjugates.
(A,B) Binding of bacteria to Leb, SLex, tumor mucin and normal mucin, using the microtiter based assay (n = 4). (C,D) Viability of bacteria after 24 h culture in the presence of Leb, SLex, tumor mucin or normal mucin samples expressed as % change in alamarBlue reduction (n = 6). Results are shown as percent of reduced alamarBlue compared to each bacteria cultured without glycoconjugates/mucins, ap ≤ 0.05, aap ≤ 0.01, aaap ≤ 0.001 ANOVA with Dunnett’s post hoc test, *p ≤ 0.05 **p ≤ 0.01, ***p ≤ 0.001 Student’s t-test. All values are mean ± S.E.M. The results in the graphs have been reproduced at least twice with the same outcome.
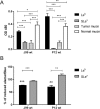
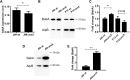
Figure 8. Effect of arsS deletion on BabA/babA expression.
(A) mRNA expression of babA in J99 wt and J99_ΔarsS_. Expression of babA after 24 h of culture in liquid medium, measured as ΔCt in relation to 16 S rRNA as a housekeeping gene. Stars indicate difference in expression compared by paired sample t-test, *p ≤ 0.05, where Ct values of each experiment were paired to remove confounding factors of variable base levels between experiments (n = 3). (B) Immunoblot detecting BabA in bacterial lysates after growth on plate. AlpB was used as a loading control. (C) Quantification of the BabA immunoblot, normalized to AlpB (n = 5–7). (D) Immunoblot detecting SabA in J99 lysates. AlpB was used as a loading control. (E) Quantification of the SabA immunoblot, normalized to AlpB (n = 5–8). Data are presented as median with interquartile range, and analyzed with the Mann-Whitney U-test (*p ≤ 0.05, **p ≤ 0.01).
Similar articles
- Human gastric mucins differently regulate Helicobacter pylori proliferation, gene expression and interactions with host cells.
Skoog EC, Sjöling Å, Navabi N, Holgersson J, Lundin SB, Lindén SK. Skoog EC, et al. PLoS One. 2012;7(5):e36378. doi: 10.1371/journal.pone.0036378. Epub 2012 May 1. PLoS One. 2012. PMID: 22563496 Free PMC article. - BabA-mediated adherence of pediatric ulcerogenic H. pylori strains to gastric mucins at neutral and acidic pH.
Quintana-Hayashi MP, Rocha R, Padra M, Thorell A, Jin C, Karlsson NG, Roxo-Rosa M, Oleastro M, Lindén SK. Quintana-Hayashi MP, et al. Virulence. 2018;9(1):1699-1717. doi: 10.1080/21505594.2018.1532243. Virulence. 2018. PMID: 30298790 Free PMC article. - Dynamics of Lewis b binding and sequence variation of the babA adhesin gene during chronic Helicobacter pylori infection in humans.
Nell S, Kennemann L, Schwarz S, Josenhans C, Suerbaum S. Nell S, et al. mBio. 2014 Dec 16;5(6):e02281-14. doi: 10.1128/mBio.02281-14. mBio. 2014. PMID: 25516619 Free PMC article. - Helicobacter pylori adhesion to gastric epithelial cells is mediated by glycan receptors.
Magalhães A, Reis CA. Magalhães A, et al. Braz J Med Biol Res. 2010 Jul;43(7):611-8. doi: 10.1590/s0100-879x2010007500049. Epub 2010 Jun 7. Braz J Med Biol Res. 2010. PMID: 20521012 Review. - [The role of gastric mucins in interactions with Helicobacter pylori].
Radziejewska I. Radziejewska I. Postepy Hig Med Dosw (Online). 2012 Jan 30;66:60-6. Postepy Hig Med Dosw (Online). 2012. PMID: 22371407 Review. Polish.
Cited by
- Mucin Glycans: A Target for Cancer Therapy.
Sun L, Zhang Y, Li W, Zhang J, Zhang Y. Sun L, et al. Molecules. 2023 Oct 11;28(20):7033. doi: 10.3390/molecules28207033. Molecules. 2023. PMID: 37894512 Free PMC article. Review. - Helicobacter pylori employs a general protein glycosylation system for the modification of outer membrane adhesins.
Teng KW, Hsieh KS, Hung JS, Wang CJ, Liao EC, Chen PC, Lin YH, Wu DC, Lin CH, Wang WC, Chan HL, Huang SK, Kao MC. Teng KW, et al. Gut Microbes. 2022 Jan-Dec;14(1):2130650. doi: 10.1080/19490976.2022.2130650. Gut Microbes. 2022. PMID: 36206406 Free PMC article. - Brachyspira Species Avidity to Colonic Mucins from Pigs with and without Brachyspira hyodysenteriae Infection Is Species Specific and Varies between Strains.
Quintana-Hayashi MP, Erhardsson M, Mahu M, Venkatakrishnan V, Haesebrouck F, Pasmans F, Lindén S. Quintana-Hayashi MP, et al. Infect Immun. 2021 Nov 16;89(12):e0048621. doi: 10.1128/IAI.00486-21. Epub 2021 Sep 20. Infect Immun. 2021. PMID: 34543117 Free PMC article. - Nanohybrid-based immunosensor prepared for Helicobacter pylori BabA antigen detection through immobilized antibody assembly with @ Pdnano/rGO/PEDOT sensing platform.
Gupta S, Jain U, Murti BT, Putri AD, Tiwari A, Chauhan N. Gupta S, et al. Sci Rep. 2020 Dec 4;10(1):21217. doi: 10.1038/s41598-020-78068-w. Sci Rep. 2020. PMID: 33277599 Free PMC article. - TFF1 Induces Aggregation and Reduces Motility of Helicobacter pylori.
Eletto D, Vllahu M, Mentucci F, Del Gaudio P, Petrella A, Porta A, Tosco A. Eletto D, et al. Int J Mol Sci. 2021 Feb 12;22(4):1851. doi: 10.3390/ijms22041851. Int J Mol Sci. 2021. PMID: 33673347 Free PMC article.
References
- Ho S. B. et al.. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology 109, 735–747 (1995). - PubMed
- Warren J. R. & Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1, 1273–1275 (1983). - PubMed
- Lindén S. et al.. Strain- and blood group-dependent binding of Helicobacter pylori to human gastric MUC5AC glycoforms. Gastroenterology 123, 1923–1930. (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical