pHisphorylation: the emergence of histidine phosphorylation as a reversible regulatory modification - PubMed (original) (raw)
Review
pHisphorylation: the emergence of histidine phosphorylation as a reversible regulatory modification
Stephen Rush Fuhs et al. Curr Opin Cell Biol. 2017 Apr.
Abstract
Histidine phosphorylation is crucial for prokaryotic signal transduction and as an intermediate for several metabolic enzymes, yet its role in mammalian cells remains largely uncharted. This is primarily caused by difficulties in studying histidine phosphorylation because of the relative instability of phosphohistidine (pHis) and lack of specific antibodies and methods to preserve and detect it. The recent synthesis of stable pHis analogs has enabled development of pHis-specific antibodies and their use has started to shed light onto this important, yet enigmatic posttranslational modification. We are beginning to understand that pHis has broader roles in protein and cellular function including; cell cycle regulation, phagocytosis, regulation of ion channel activity and metal ion coordination. Two mammalian histidine kinases (NME1 and NME2), two pHis phosphatases (PHPT1 and LHPP), and a handful of substrates were previously identified. These new tools have already led to the discovery of an additional phosphatase (PGAM5) and hundreds of putative substrates. New methodologies are also being developed to probe the pHis phosphoproteome and determine functional consequences, including negative ion mode mass spectroscopy and unnatural amino acid incorporation. These new tools and strategies have the potential to overcome the unique challenges that have been holding back our understanding of pHis in cell biology.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
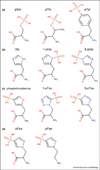
Figure 1. pHis Isomers and Structural Analogs
Structural drawings of (A) the phosphoester amino acids; pSer, pThr and pTyr are contrasted with (B) histidine, 3-phosphohistidine (3-pHis) and 1-phosphohistidine (1-pHis). Examples of phosphohistidine structural analogs designed for antibody generation include; (C) phosphofurylalanine and the two phosphoryltriazolylalanine analogs (3-pTza) and (1-pTza). (D) Second-generation, pyrazole-based pHis analogs; 4-Phosphopyrazol-2-yl alanine (pPza) and phosphono-pyrazolyl ethylamine (pPye).
Figure 2. Summary of pHis Cellular Functions
An illustration of the pHis related proteins discussed in this review and their various functions, enzymatic reactions and subcellular localizations. NME1/2 protein histidine kinase functions are in yellow, pHis enzyme intermediates are in green, phosphohistidine phosphatases are in white and pHis substrates are in bold. Beneath each protein’s gene name is the specific amino acid position number of the pHis residue in red. Cellular functions of specific pHis proteins are in blue. The subcellular localization of pHis related proteins and functions are in grey. Curved arrows represent reactions catalyzed by enzymes that utilize pHis intermediates. For LHPP, phospholysine and 3-phsphohistidine are substrates in vitro, however no known substrates have yet been identified in vivo.
Similar articles
- Histidine Phosphorylation: Protein Kinases and Phosphatases.
Ning J, Sala M, Reina J, Kalagiri R, Hunter T, McCullough BS. Ning J, et al. Int J Mol Sci. 2024 Jul 21;25(14):7975. doi: 10.3390/ijms25147975. Int J Mol Sci. 2024. PMID: 39063217 Free PMC article. Review. - Chasing phosphohistidine, an elusive sibling in the phosphoamino acid family.
Kee JM, Muir TW. Kee JM, et al. ACS Chem Biol. 2012 Jan 20;7(1):44-51. doi: 10.1021/cb200445w. Epub 2011 Dec 9. ACS Chem Biol. 2012. PMID: 22148577 Free PMC article. Review. - The many ways that nature has exploited the unusual structural and chemical properties of phosphohistidine for use in proteins.
Kalagiri R, Hunter T. Kalagiri R, et al. Biochem J. 2021 Oct 15;478(19):3575-3596. doi: 10.1042/BCJ20210533. Biochem J. 2021. PMID: 34624072 Free PMC article. Review. - Analysis of 1- and 3-Phosphohistidine (pHis) Protein Modification Using Model Enzymes Expressed in Bacteria.
Clubbs Coldron AKM, Byrne DP, Eyers PA. Clubbs Coldron AKM, et al. Methods Mol Biol. 2020;2077:63-81. doi: 10.1007/978-1-4939-9884-5_5. Methods Mol Biol. 2020. PMID: 31707652 - A journey from phosphotyrosine to phosphohistidine and beyond.
Hunter T. Hunter T. Mol Cell. 2022 Jun 16;82(12):2190-2200. doi: 10.1016/j.molcel.2022.05.007. Epub 2022 Jun 1. Mol Cell. 2022. PMID: 35654043 Free PMC article. Review.
Cited by
- Strategies for mass spectrometry-based phosphoproteomics using isobaric tagging.
Liu X, Fields R, Schweppe DK, Paulo JA. Liu X, et al. Expert Rev Proteomics. 2021 Sep;18(9):795-807. doi: 10.1080/14789450.2021.1994390. Epub 2021 Oct 28. Expert Rev Proteomics. 2021. PMID: 34652972 Free PMC article. Review. - Structural basis for differential recognition of phosphohistidine-containing peptides by 1-pHis and 3-pHis monoclonal antibodies.
Kalagiri R, Stanfield RL, Meisenhelder J, La Clair JJ, Fuhs SR, Wilson IA, Hunter T. Kalagiri R, et al. Proc Natl Acad Sci U S A. 2021 Feb 9;118(6):e2010644118. doi: 10.1073/pnas.2010644118. Proc Natl Acad Sci U S A. 2021. PMID: 33547238 Free PMC article. - Phosphorylation and sulfation share a common biosynthetic pathway, but extend biochemical and evolutionary diversity of biological macromolecules in distinct ways.
Lima MA, Rudd TR, Fernig DG, Yates EA. Lima MA, et al. J R Soc Interface. 2022 Aug;19(193):20220391. doi: 10.1098/rsif.2022.0391. Epub 2022 Aug 3. J R Soc Interface. 2022. PMID: 35919982 Free PMC article. - NME3 is a gatekeeper for DRP1-dependent mitophagy in hypoxia.
Chen CW, Su C, Huang CY, Huang XR, Cuili X, Chao T, Fan CH, Ting CW, Tsai YW, Yang KC, Yeh TY, Hsieh ST, Chen YJ, Feng Y, Hunter T, Chang ZF. Chen CW, et al. Nat Commun. 2024 Mar 13;15(1):2264. doi: 10.1038/s41467-024-46385-7. Nat Commun. 2024. PMID: 38480688 Free PMC article. - The actions of NME1/NDPK-A and NME2/NDPK-B as protein kinases.
Attwood PV, Muimo R. Attwood PV, et al. Lab Invest. 2018 Mar;98(3):283-290. doi: 10.1038/labinvest.2017.125. Epub 2017 Dec 4. Lab Invest. 2018. PMID: 29200201 Review.
References
- Boyer PD, DeLuca M, Ebner KE, Hultquist DE, Peter JE. Identification of phosphohistidine in digests from a probable intermediate of oxidative phosphorylation. J Biol Chem. 1962;237:3306–3308. - PubMed
- Attwood PV, Piggott MJ, Zu XL, Besant PG. Focus on phosphohistidine. Amino Acids. 2007;32:145–156. - PubMed
- Fuhs SR, Meisenhelder J, Aslanian A, Ma L, Zagorska A, Stankova M, Binnie A, Al-Obeidi F, Mauger J, Lemke G, et al. Monoclonal 1- and 3-phosphohistidine antibodies: new tools to study histidine phosphorylation. Cell. 2015;162:198–210. This study describes the generation and use of monoclonal antibodies that specifically recongnize either the 1-pHis or 3-pHis isomer. Their characterization and specificity is demonstrated and methods for their use in several immunological assays including immunoblotting, immunofluorescence and immunoaffity purification of pHis proteins and analysis by LC-MS/MS.
- Matthews HR, Chan K. Protein histidine kinase. Methods Mol Biol. 2001;124:171–182. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous