Nuclear Pore-Like Structures in a Compartmentalized Bacterium - PubMed (original) (raw)
. 2017 Feb 1;12(2):e0169432.
doi: 10.1371/journal.pone.0169432. eCollection 2017.
Amanda Nouwens 1, Richard I Webb 2, Kathryn Green 2, Benjamin Yee 1, Garry Morgan 2, Andrew Leis 3, Kuo-Chang Lee 1, Margaret K Butler 1, Nicholas Chia 4, Uyen Thi Phuong Pham 1, Stinus Lindgreen 5, Ryan Catchpole 5 6, Anthony M Poole 5 6 7 8, John A Fuerst 1
Affiliations
- PMID: 28146565
- PMCID: PMC5287468
- DOI: 10.1371/journal.pone.0169432
Nuclear Pore-Like Structures in a Compartmentalized Bacterium
Evgeny Sagulenko et al. PLoS One. 2017.
Abstract
Planctomycetes are distinguished from other Bacteria by compartmentalization of cells via internal membranes, interpretation of which has been subject to recent debate regarding potential relations to Gram-negative cell structure. In our interpretation of the available data, the planctomycete Gemmata obscuriglobus contains a nuclear body compartment, and thus possesses a type of cell organization with parallels to the eukaryote nucleus. Here we show that pore-like structures occur in internal membranes of G.obscuriglobus and that they have elements structurally similar to eukaryote nuclear pores, including a basket, ring-spoke structure, and eight-fold rotational symmetry. Bioinformatic analysis of proteomic data reveals that some of the G. obscuriglobus proteins associated with pore-containing membranes possess structural domains found in eukaryote nuclear pore complexes. Moreover, immunogold labelling demonstrates localization of one such protein, containing a β-propeller domain, specifically to the G. obscuriglobus pore-like structures. Finding bacterial pores within internal cell membranes and with structural similarities to eukaryote nuclear pore complexes raises the dual possibilities of either hitherto undetected homology or stunning evolutionary convergence.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
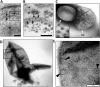
Fig 1. Pores are inserted into the internal membranes of Gemmata obscuriglobus cells.
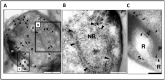
(A) Transmission electron micrograph of a thin-section of a cryosubstituted cell of G. obscuriglobus, showing a portion of the nuclear body envelope, apparently consisting of two closely apposed membranes enclosing the fibrillar nucleoid DNA (N) (for evidence of DNA fibrillar nature in G. obscuriglobus see [9]. The membranes (arrows) are interrupted by a disc-like structure (indicated by arrowhead within the boxed region) consistent with a pore complex inserted between the membranes on either side. Bar, 50 nm. (B) Enlargement of the sectioned cell of G. obscuriglobus seen in Fig 1A, showing a disc structure (arrowhead) seen en face, situated between the folded double membranes of the nuclear body envelope on either side (arrows). Bar, 50 nm. (C) Transmission electron micrograph of cell lysed by grinding in liquid N2, followed by negative staining of thawed cells with uranyl acetate. An internal membrane fragment (IM) possibly representing the nuclear body envelope or other internal compartment membranes appears to have been released from a lysed cell, and the mostly intact cell wall (CW) can also be seen. The membrane displays numerous evenly distributed pore structures on its surface, enlarged views of which can be seen in the inset. Bar, 500 nm. Inset shows enlargement of pore structures, which display a dense core surrounded by a light ring further surrounded by a dense ring. Bar, 50 nm. (D) Transmission electron micrograph of negatively stained preparation of a completely released internal compartment from cells lysed as in C. Pore structures are widely distributed over the membrane surface including within the boxed region. The ‘canoe’ shape is typical for pore-containing membranes. Bar, 500 nm. (E) An enlarged view of the boxed region in Fig 1D showing the large pore structures (arrows), each displaying dark pore centre regions, and lighter inner and outer ring structures, distributed densely on the membrane surface. Bar, 100 nm.
Fig 2. Gemmata obscuriglobus internal membrane pores as seen in freeze-fractured cells.
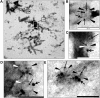
(A) Transmission electron micrograph of a platinum/carbon (Pt/C)-shadowed replica of a whole cell of G. obscuriglobus which has been prepared via the freeze-fracture technique. Bar, 100 nm. Inside the cell, a large spherical internal organelle consistent with the nuclear body organelle surrounding the nucleoid has been fractured (split) along and through the surface membranes of its envelope. Pores with a central core and at least one surrounding ring are visible on one region of one of the membranes of this organelle surface. Insets represent successive enlarged views of the boxed region in the main image displaying the pores at higher magnification. Bars, 100nm. At the highest enlargement the substructure of each of several pores can be resolved including central core and surrounding inner dark and outer light rings (right inset). (B) This micrograph of the whole cell reveals an apparently cross-fractured major internal organelle compartment and a membrane surface (boxed) representing a fracture through the membrane surrounding the organelle. Bar, 200 nm. (C) An enlarged view of the boxed region of the freeze-fractured cell seen in Fig 2B showing a region of a membrane surface where roughly circular pore structures (arrowheads) are visible, in some cases with two light rings surrounding a dark centre,. Bar, 50 nm.
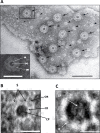
Fig 3. Pores in the membranes of Gemmata obscuriglobus released via sonication.
(A) Transmission electron micrograph of a membrane fragment released from a lysed cell via sonication and negatively stained with ammonium molybdate. Large pores (arrows) with relatively electron-dense pore centers surrounded by a thin lighter inner ring and a thicker outer ring are seen. Smaller pore structures (arrowheads) are also visible and may represent either another class of pores or a result of a reverse view of the same large pores resulting from overlapping folds in the membrane (evidence for such structures is not derived from other microscopy methods). Bar, 100 nm. Inset: enlargement of boxed large pore in main Fig where a pore centre (PC), an inner ring (IR) and an outer ring (OR) can be distinguished. Bar, 50 nm. (B) TEM of a pore seen in negatively stained membrane fraction isolated from sonication-lysed cells, showing pore complex structure including outer ring (OR), inner ring (IR), spokes connecting inner and outer rings (S) and central plug (CP). Bar, 30 nm. (C) Enlarged view of the inner ring (IR) and central plug (CP) of the boxed pores in Fig 3A, the octagonal shape of the rings (especially visible if the outer edge of the outer ring is traced) is consistent with an eight-fold symmetry. Bar, 15 nm.
Fig 4. Architecture of the Gemmata obscuriglobus pore.
(A) Structure of pores embedded into membranes from fraction 3 purified via density gradient centrifugations and visualized via TEM of thin-sections. The spiral seen consists of a membrane (arrows) in which pores are embedded interrupting dense-light-dense layers of the trilaminar membrane. Basket structures (arrowheads) of each pore complex project only from one side of the membrane. The inner and outer dense leaflets of the membrane are seen to be connected forming a continuous folded membrane (on each side of the pore) of extreme membrane curvature (arrows). Bar, 100 nm. (See also S1 and S2 Videos for 3D reconstructions of the membranes and S3 Video for 3D reconstruction of the pore). (B) Transmission electron micrographs from a tilt-series of one pore. In panel 1 intact membrane without pore is seen, while in panels 2–6 passing through progressive slices generated via the tilt-series, the pore appears, interrupting the trilaminar membrane on either side, and most clearly indicated by a basket structure projecting below the plane of the membrane (arrowhead). In panels 3 and 4, the central plug region of the pore can be seen (arrows). In panel 6 the trilaminar membrane is again continuous, but some parts of the basket structure are still visible (arrow). This series is consistent with the interruption of membrane by embedded pore structures, the basket component of which projects beyond the membrane plane. Bar, 20 nm. (C) Micrograph from cryo-EM of a frozen-hydrated preparation of the isolated and sucrose-purified fraction 3 membranes. Two randomly selected pores (see S10 Fig supplement 1for cryo-EM of fraction 3 membrane sheets from which these pores were selected) clearly display inner ring (IR), outer ring (OR) and central plug (CP). This image has been processed via uniform application of a conservative bandpass filter (respective low- and high-frequency cut-offs of 40 and 3 pixels). Bar, 30 nm. (D) Modified Markham rotation analysis of one of the pores from Fig 4C showing reinforcement of 8-fold symmetry of pore structure. Bar, 10 nm.
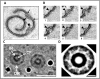
Fig 5. 3-D reconstructions of the pore complex.
(A and B) Views of the 3-D reconstructions based on one spiral membrane from fraction 3 membranes (see Fig 4A). Pore complexes (arrows) are visible as embedded structures in the surface of the envelope, shown as viewed from the inner side of the spiral in Fig 5A and from the outer side in Fig 5B. Fig 5C shows the basket structure of one of these pores projecting from the inner side of the membrane spiral. Bars, 20 nm. (D and E) Reconstruction of architecture of a single pore seen from two different angles. In panel D, a side view of the pore displays the basket structure with its distal ring (arrowhead) and a series of struts (arrow) connecting with the main pore rings. In panel E, a top view shows the ring-like element (arrowhead) of the main part of the pore and a central plug structure is visible within the pore connected to the ring’s inner rim via spokes.
Fig 6. Model of the pore complex of Gemmata obscuriglobus.
The pore complex is composed of at least two concentric upper rings (blue), and a lower ring (light blue) connected by struts to a distal ring (green) to form a basket structure. The central plug (purple) rests within the inner ring and spans the length of the pore. The whole pore complex rests within membrane (orange). The structure and dimensions are based on available data from all EM methods applied, from both whole cells and fraction 3 isolated membranes, and with minimal extrapolation, so that although the pore is probably not a hollow structure the space within the pore has not been filled in.
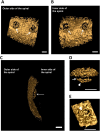
Fig 7. Protein composition of Gemmata obscuriglobus pore-containing membrane.
(A) SDS-PAGE gel showing that G.obscuriglobus cells have three different types of membranes. Exclusively pore-containing membranes (fraction 3) display a characteristic protein profile distinct from that of membrane fractions which do not possess pore structures. (B) Venn diagram showing the number and distribution of proteins among the fractions and (in brackets) the number of proteins with the beta-propeller folds. The members of the beta-propeller cluster belong either exclusively to fraction 3 (4 proteins), or to fractions 3 and 2 (2 proteins), and to fractions 2, 3 and 6 (2 proteins). No beta-propeller containing proteins were found exclusively in fractions 2 or 6. (C) A beta-propeller family found in fraction 3 (pore-containing membranes), including some exclusive to fraction 3. Cluster analyses revealed a set of proteins with conserved C-terminal regions (S13–S16 Figs) that model beta-propeller folds with high (>95%) confidence. Models 3 (for protein ZP_02737072), 4 (ZP_02736670), 5 (ZP_02734776) and 6 (ZP_ZP_02734577) were deduced from proteins found exclusively in fraction 3 (pore-containing fraction); models 2 (for ZP_02737073) and 7 (for ZP_02733245) were deduced from proteins found in fractions 3 and 2 only; models 1 (for ZP_02737797) and 8 (for ZP_02731113)–for proteins found in fractions 3, 2, and 6 (S4 Table).
Fig 8. The antibody 6670 recognises internal membranes in G.obscuriglobus cells.
**_(A)_**TEM of a thin-sectioned cryosubstituted cell labelled with the antibody 6670. The majority of the gold particles (arrows) are seen to be bound to intracytoplasmic membrane (ICM, arrowheads). This membrane separates the electron-dense ribosome-free paryphoplasm (P) from relatively electron-transparent riboplasm (R), as well as riboplasm vesicles from each other. A few particles label the border envelope between NB and riboplasm including double membrane regions. Bar, 500nm. (B) An enlarged view of the boxed region B in Fig 8A showing the nuclear body (NB) with nucleoid DNA. A few gold particles (arrows) are visible on the envelope membranes (arrowheads), separating NB and riboplasm. Bar, 200 nm. (C) An enlarged view of the boxed region C in Fig 8A showing an electron-transparent region continuous with riboplasm, surrounded by paryphoplasm which is separated from the riboplasm-continuous region by ICM. Gold particles (arrows) unambiguously label the ICM (arrowheads). Bar, 200 nm.
Fig 9. The antibody 6670 recognises pores in the isolated membranes.
(A) Immunogold labelling of membrane sheets from membrane fraction 3 with the antibody 6670. Pellets of such fractionated membrane sheets were cryosubstituted using a protocol suitable for antigen preparation and sectioned before immunolabelling (note that the preservation of structure is not optimal in such sections due to the need for less harsh cryosubstitution conditions to allow preservation of antigenic epitopes for antibody recognition). In the majority of cases the gold particles indicating antibody can be seen as associated with the outer ring of the pores, which can be recognized in each case by a characteristic circular ring structure surrounding an electron-dense plug at their centre. Panels (B, C, D, and E) show enlarged areas of (A), which are marked as boxes in (A). In all the cases the gold particles can be seen at the edge of the pores. In some cases more than one gold particle is associated with the pores (for example see box (D), the bottom pore which is surrounded by three particles). For statistical analyses approximately the same areas were used for counting the particles: 397 particles were observed as associated with pores (distance from a pore does not exceed 20nm) and 45 particles were considered as not associated. Short thick black arrows indicate gold particles, black or white arrowheads (depending on background)–pores, and thin longer black arrows indicate the electron-dense core defining the centre of each labelled pore. Bars, A– 1 μm, B -200nm, C, D, and E– 100nm.
Similar articles
- Structural studies of planctomycete Gemmata obscuriglobus support cell compartmentalisation in a bacterium.
Sagulenko E, Morgan GP, Webb RI, Yee B, Lee KC, Fuerst JA. Sagulenko E, et al. PLoS One. 2014 Mar 14;9(3):e91344. doi: 10.1371/journal.pone.0091344. eCollection 2014. PLoS One. 2014. PMID: 24632833 Free PMC article. - Three-dimensional reconstruction of bacteria with a complex endomembrane system.
Santarella-Mellwig R, Pruggnaller S, Roos N, Mattaj IW, Devos DP. Santarella-Mellwig R, et al. PLoS Biol. 2013;11(5):e1001565. doi: 10.1371/journal.pbio.1001565. Epub 2013 May 21. PLoS Biol. 2013. PMID: 23700385 Free PMC article. - Nested bacterial boxes: nuclear and other intracellular compartments in planctomycetes.
Fuerst JA, Sagulenko E. Fuerst JA, et al. J Mol Microbiol Biotechnol. 2013;23(1-2):95-103. doi: 10.1159/000346544. Epub 2013 Apr 18. J Mol Microbiol Biotechnol. 2013. PMID: 23615198 Review. - The cell cycle of the planctomycete Gemmata obscuriglobus with respect to cell compartmentalization.
Lee KC, Webb RI, Fuerst JA. Lee KC, et al. BMC Cell Biol. 2009 Jan 14;10:4. doi: 10.1186/1471-2121-10-4. BMC Cell Biol. 2009. PMID: 19144151 Free PMC article. - Intracellular compartmentation in planctomycetes.
Fuerst JA. Fuerst JA. Annu Rev Microbiol. 2005;59:299-328. doi: 10.1146/annurev.micro.59.030804.121258. Annu Rev Microbiol. 2005. PMID: 15910279 Review.
Cited by
- The Planctomycetia: an overview of the currently largest class within the phylum Planctomycetes.
Vitorino IR, Lage OM. Vitorino IR, et al. Antonie Van Leeuwenhoek. 2022 Feb;115(2):169-201. doi: 10.1007/s10482-021-01699-0. Epub 2022 Jan 17. Antonie Van Leeuwenhoek. 2022. PMID: 35037113 Review. - Response: Commentary: Manifold Routes to a Nucleus.
Poole AM, Hendrickson HL. Poole AM, et al. Front Microbiol. 2019 Nov 8;10:2585. doi: 10.3389/fmicb.2019.02585. eCollection 2019. Front Microbiol. 2019. PMID: 31781075 Free PMC article. No abstract available. - Editorial: Structure, Function and Evolution of Complex Cellular Organization in Bacteria and Archaea.
Franke JD, Fuerst JA, Poole AM. Franke JD, et al. Front Microbiol. 2021 Aug 30;12:751416. doi: 10.3389/fmicb.2021.751416. eCollection 2021. Front Microbiol. 2021. PMID: 34526983 Free PMC article. No abstract available. - The Phage Nucleus and Tubulin Spindle Are Conserved among Large Pseudomonas Phages.
Chaikeeratisak V, Nguyen K, Egan ME, Erb ML, Vavilina A, Pogliano J. Chaikeeratisak V, et al. Cell Rep. 2017 Aug 15;20(7):1563-1571. doi: 10.1016/j.celrep.2017.07.064. Cell Rep. 2017. PMID: 28813669 Free PMC article. - Manifold Routes to a Nucleus.
Hendrickson HL, Poole AM. Hendrickson HL, et al. Front Microbiol. 2018 Oct 26;9:2604. doi: 10.3389/fmicb.2018.02604. eCollection 2018. Front Microbiol. 2018. PMID: 30416499 Free PMC article.
References
MeSH terms
Substances
Grants and funding
JAF was supported by Australian Research Council Discovery Project DP0881485. AMP was supported by Royal Society of New Zealand RDF-UOC1101. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
LinkOut - more resources
Full Text Sources
Other Literature Sources