Bacterial Virulence Factors: Secreted for Survival - PubMed (original) (raw)
Review
Bacterial Virulence Factors: Secreted for Survival
Aditya Kumar Sharma et al. Indian J Microbiol. 2017 Mar.
Abstract
Virulence is described as an ability of an organism to infect the host and cause a disease. Virulence factors are the molecules that assist the bacterium colonize the host at the cellular level. These factors are either secretory, membrane associated or cytosolic in nature. The cytosolic factors facilitate the bacterium to undergo quick adaptive-metabolic, physiological and morphological shifts. The membrane associated virulence factors aid the bacterium in adhesion and evasion of the host cell. The secretory factors are important components of bacterial armoury which help the bacterium wade through the innate and adaptive immune response mounted within the host. In extracellular pathogens, the secretory virulence factors act synergistically to kill the host cells. In this review, we revisit the role of some of the secreted virulence factors of two human pathogens: _Mycobacterium tuberculosis_-an intracellular pathogen and _Bacillus anthracis_-an extracellular pathogen. The advances in research on the role of secretory factors of these pathogens during infection are discussed.
Keywords: Anthrax; Bacillus; Mycobacterium; Phosphatases; Tuberculosis; Virulence.
Figures
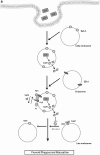
Fig. 1
Secretory phosphatase- SapM and SerB2 of Mycobacterium tuberculosis. a SapM is secreted by Mtb and enters host cells by an unknown mechanism. SapM dephosphorylates PI3P causing reduction of PI3P concentration on the phagosomal membrane. This in turn inhibits the recruitment of EEA1 protein, preventing phagosomal maturation. Additionally, SapM has been shown to block Rab7 through its CT domain, resulting in the inhibition of phagosomal maturation into a phagolysosome. b SerB2 dephosphorylates MAPK-p38 and NFκB p65 to suppress IL-8 synthesis in macrophages. SerB2 also dephosphorylates cofilin leading to its activation causing cytoskeleton remodelling
Fig. 1
Secretory phosphatase- SapM and SerB2 of Mycobacterium tuberculosis. a SapM is secreted by Mtb and enters host cells by an unknown mechanism. SapM dephosphorylates PI3P causing reduction of PI3P concentration on the phagosomal membrane. This in turn inhibits the recruitment of EEA1 protein, preventing phagosomal maturation. Additionally, SapM has been shown to block Rab7 through its CT domain, resulting in the inhibition of phagosomal maturation into a phagolysosome. b SerB2 dephosphorylates MAPK-p38 and NFκB p65 to suppress IL-8 synthesis in macrophages. SerB2 also dephosphorylates cofilin leading to its activation causing cytoskeleton remodelling
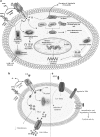
Fig. 2
Schematic diagram explaining the physiological imbalances observed in the host cells by anthrax major secreted virulence factors, which are lethal toxin (LT), edema toxin (ET), and anthrolysin O (AL)
Similar articles
- Host-pathogen interactions during Mycobacterium tuberculosis infections.
Stanley SA, Cox JS. Stanley SA, et al. Curr Top Microbiol Immunol. 2013;374:211-41. doi: 10.1007/82_2013_332. Curr Top Microbiol Immunol. 2013. PMID: 23881288 Review. - Comparative analysis of virulence factors secreted by Bacillus anthracis Sterne at host body temperature.
Kim SK, Shahid S, Kim SH, Park JH, Lee HT, Jung KH, Chai YG. Kim SK, et al. Lett Appl Microbiol. 2012 Apr;54(4):306-12. doi: 10.1111/j.1472-765X.2012.03209.x. Epub 2012 Feb 15. Lett Appl Microbiol. 2012. PMID: 22268495 - [Advances in understanding of the virulence mechanism of Mycobacterium tuberculosis].
Kawamura I. Kawamura I. Nihon Hansenbyo Gakkai Zasshi. 2008 Sep;77(3):219-24. doi: 10.5025/hansen.77.219. Nihon Hansenbyo Gakkai Zasshi. 2008. PMID: 18800644 Review. Japanese. - A New ESX-1 Substrate in Mycobacterium marinum That Is Required for Hemolysis but Not Host Cell Lysis.
Bosserman RE, Nicholson KR, Champion MM, Champion PA. Bosserman RE, et al. J Bacteriol. 2019 Jun 21;201(14):e00760-18. doi: 10.1128/JB.00760-18. Print 2019 Jul 15. J Bacteriol. 2019. PMID: 30833360 Free PMC article. - Immune system paralysis by anthrax lethal toxin: the roles of innate and adaptive immunity.
Fukao T. Fukao T. Lancet Infect Dis. 2004 Mar;4(3):166-70. doi: 10.1016/S1473-3099(04)00940-5. Lancet Infect Dis. 2004. PMID: 14998502 Review.
Cited by
- Transcriptome Analysis Reveals Modulation of Human Stem Cells from the Apical Papilla by Species Associated with Dental Root Canal Infection.
Razghonova Y, Zymovets V, Wadelius P, Rakhimova O, Manoharan L, Brundin M, Kelk P, Romani Vestman N. Razghonova Y, et al. Int J Mol Sci. 2022 Nov 20;23(22):14420. doi: 10.3390/ijms232214420. Int J Mol Sci. 2022. PMID: 36430898 Free PMC article. - High-Throughput Transcriptomic Profiling Reveals the Inhibitory Effect of Hydroquinine on Virulence Factors in Pseudomonas aeruginosa.
Rattanachak N, Weawsiangsang S, Daowtak K, Thongsri Y, Ross S, Ross G, Nilsri N, Baldock RA, Pongcharoen S, Jongjitvimol T, Jongjitwimol J. Rattanachak N, et al. Antibiotics (Basel). 2022 Oct 19;11(10):1436. doi: 10.3390/antibiotics11101436. Antibiotics (Basel). 2022. PMID: 36290094 Free PMC article. - Bacillus anthracis chain length, a virulence determinant, is regulated by membrane localized serine/threonine protein kinase PrkC.
Dhasmana N, Kumar N, Gangwal A, Keshavam CC, Singh LK, Sangwan N, Nashier P, Biswas S, Pomerantsev AP, Leppla SH, Singh Y, Gupta M. Dhasmana N, et al. J Bacteriol. 2021 Jun 1;203(11):e00582-20. doi: 10.1128/JB.00582-20. Epub 2021 Mar 22. J Bacteriol. 2021. PMID: 33753466 Free PMC article. - Infectious Foci, Comorbidities and Its Influence on the Outcomes of Septic Critically Ill Patients.
Oliveira AM, Oliveira A, Vidal R, Gonçalves-Pereira J. Oliveira AM, et al. Microorganisms. 2024 Aug 18;12(8):1705. doi: 10.3390/microorganisms12081705. Microorganisms. 2024. PMID: 39203547 Free PMC article. - The Mycoplasma spp. 'Releasome': A New Concept for a Long-Known Phenomenon.
Gaurivaud P, Tardy F. Gaurivaud P, et al. Front Microbiol. 2022 Apr 15;13:853440. doi: 10.3389/fmicb.2022.853440. eCollection 2022. Front Microbiol. 2022. PMID: 35495700 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources