Nanomaterials in Targeting Cancer Stem Cells for Cancer Therapy - PubMed (original) (raw)
Review
Nanomaterials in Targeting Cancer Stem Cells for Cancer Therapy
Weiwei Qin et al. Front Pharmacol. 2017.
Abstract
Cancer stem cells (CSCs) have been identified in almost all cancers and give rise to metastases and can also act as a reservoir of cancer cells that may cause a relapse after surgery, radiation, or chemotherapy. Thus they are obvious targets in therapeutic approaches and also a great challenge in cancer treatment. The threat presented by CSCs lies in their unlimited proliferative ability and multidrug resistance. These findings have necessitated an effective novel strategy to target CSCs for cancer treatment. Nanomaterials are on the route to providing novel methods in cancer therapies. Although, there have been a large number of excellent work in the field of targeted cancer therapy, it remains an open question how nanomaterials can meet future demands for targeting and eradicating of CSCs. In this review, we summarized recent and highlighted future prospects for targeting CSCs for cancer therapies by using a variety of nanomaterials.
Keywords: cancer stem cells; multidrug resistance; nanomaterials; targeted therapies; unlimited proliferation.
Figures
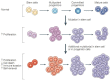
Figure 1
Cancer stem cells and tumor progression. Normal stem cells give rise to multipotent progenitor cells, committed progenitors and mature, differentiated cells. Mutations in a stem cell give rise to a stem cell with aberrant proliferation and result in a pre-malignant lesion. Additional mutations lead to the acquisition of further increased proliferation, decreased apoptosis, evasion of the immune system, and further expansion of the stem-cell compartment that is typical of malignant tumors (Dean et al., 2005).
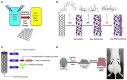
Scheme 1
The mechanisms of the engineered nanoparticles for drug delivery in cancer stem cell therapy. A summary of nanoparticles that have been explored as carriers for drug delivery in cancer stem cell therapy, together with illustrations of biophysicochemical properties.
Figure 2
(A) Graphene oxide (GO): Targeting cancer stem cells with differentiation-based nano-therapy. The current mechanistic studies suggest that GO could directly be used as a therapeutic for targeting cancer stem cells (CSCs), because of its ability to induce differentiation. In this context, we might envision that GO could be used to clear residual CSCs, with the aim of preventing tumor recurrence and distant metastasis, thereby providing a practical means for achieving “differentiation-based nano-therapy” (Fiorillo et al., 2015); (B) Schematic illustration of the preparation process of SAL-SWNTs-CHI-HA [chitosan(CHI) coated single wall carbon nanotubes (SWNTs) loaded with salinomycin (SAL) functionalized with hyaluronic acid (HA); Yao et al., 2014]; (C) Schematic diagram illustrating the concept of functionalized SWCNTs as drug carriers (Al Faraj et al., 2016b); (D) Left: schematic model showing surface and chemical structure of (ND) and Epirubicin (Epi), synthesis and aggregation of Epirubicin-nanodiamond complex (EPND). ND represented in truncated octahedron structure with different surface charge denoted with color. ND surface functional group indicated, including benzene ring, carboxyl group, and hydrogen group. Molecular skeleton representing carbon, oxygen and nitrogen atoms in Epi molecule was shown in red. Synthesis of EPND was performed under basic condition of 2.5 mM NaOH through physical adsorption between Epi and ND. Aggregation around 90 nm was formed after EPND synthesis. Right: representative image shows that EPND can inhibit tumor-initiation in murine hepatic tumor allografts (Wang et al., 2014).
Figure 3
(A) DNA origami and doxorubicin origami delivery system assembly. The long single-strand M13mp18 genomic DNA scaffold strand (blue) is folded into the triangle and tube structures through the hybridization of rationally designed staple strands. Watson−Crick base pairs in the double helices serve as docking sites for doxorubicin intercalation. After incubation with doxorubicin, the drug-loaded DNA nanostructure delivery vessels were administered to human breast cancer cell line MCF 7 cells, and the effects were investigated (Jiang et al., 2012); (B) Top: Transmission electron microscope (TEM) images of the designed DNA origami structure; Bottom: The number of viable cells subjecting to free daunorubicin or daunorubicin-loaded Horse nanostructures for 24 h (Halley et al., 2016). *p < 0.05.
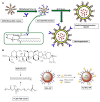
Figure 4
(A) miR-182 or Co-miR–RNA duplexes were hybridized to citrate stabilized gold nanoparticles (Au NPs) via thiol-gold bond and passivated with polyethylene glycol-Thiol (mPEG-SH) to obtain miR-182-based spherical nucleic acids (182-SNAs); (B) Analysis of tumor burden by weight and (C) bioluminescence imaging; (A–C) (Kouri et al., 2015); (D) Top: fabrication of polyelectrolyte conjugated Au NRs and drug loading; Bottom: schematic illustration of selective elimination of breast cancer stem cells (CSCs) by polyelectrolyte conjugated gold nanorods (Au NRs) mediated hyperthermia. CTAB, cetyltriethylammnonium bromide; PAA, Poly(acrylic acid); PDC, poly-diallyldimethylammonium chloride; ALDH+, aldehyde dehydrogenase positive (Xu et al., 2014).
Figure 5
(A) Drug–gold nanorods and siRNA–gold nanospheres doped in implantable hydrogels for local drug/gene delivery and local hyperthermia (Conde et al., 2016). (B) Schematic illustration of the preparation of biocompatible porous silicon nanoparticles@gold nanorods@double emulsion (PSi NPs@AuNRs@double emulsion) co-delivery platform for co-loading versatile therapeutics, DNA origami, antibody, and hydrophobic functional PSi NPs loaded with Erlotinib or Afatinib (Kong et al., 2016).
Figure 6
(A) Post-insertion method for the preparation of CD44-doxil (Arabi et al., 2015). (B) The preparation procedure of salinomycin-loaded PEGylated poly(lactic-co-glycolic acid) nanoparticles (SAL-NP) or SAL-NP linked with CD133 aptamers (Ap-SAL-NP; Ni et al., 2015).
Similar articles
- Nanomaterials for targeted drug delivery to cancer stem cells.
Orza A, Casciano D, Biris A. Orza A, et al. Drug Metab Rev. 2014 May;46(2):191-206. doi: 10.3109/03602532.2014.900566. Epub 2014 Apr 4. Drug Metab Rev. 2014. PMID: 24697156 Review. - Natural Compounds Targeting Cancer Stem Cells: A Promising Resource for Chemotherapy.
Das PK, Zahan T, Abdur Rakib M, Khanam JA, Pillai S, Islam F. Das PK, et al. Anticancer Agents Med Chem. 2019;19(15):1796-1808. doi: 10.2174/1871520619666190704111714. Anticancer Agents Med Chem. 2019. PMID: 31272363 Review. - Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways.
Leon G, MacDonagh L, Finn SP, Cuffe S, Barr MP. Leon G, et al. Pharmacol Ther. 2016 Feb;158:71-90. doi: 10.1016/j.pharmthera.2015.12.001. Epub 2015 Dec 17. Pharmacol Ther. 2016. PMID: 26706243 Review. - Therapeutic approaches targeting cancer stem cells.
Pan Y, Ma S, Cao K, Zhou S, Zhao A, Li M, Qian F, Zhu C. Pan Y, et al. J Cancer Res Ther. 2018;14(7):1469-1475. doi: 10.4103/jcrt.JCRT_976_17. J Cancer Res Ther. 2018. PMID: 30589025 Review. - Targeting head and neck tumoral stem cells: From biological aspects to therapeutic perspectives.
Méry B, Guy JB, Espenel S, Wozny AS, Simonet S, Vallard A, Alphonse G, Ardail D, Rodriguez-Lafrasse C, Magné N. Méry B, et al. World J Stem Cells. 2016 Jan 26;8(1):13-21. doi: 10.4252/wjsc.v8.i1.13. World J Stem Cells. 2016. PMID: 26839637 Free PMC article. Review.
Cited by
- Bioactive Immunomodulatory Compounds: A Novel Combinatorial Strategy for Integrated Medicine in Oncology? BAIC Exposure in Cancer Cells.
Corradetti B, Vaiasicca S, Mantovani M, Virgili E, Bonucci M, Hammarberg Ferri I. Corradetti B, et al. Integr Cancer Ther. 2019 Jan-Dec;18:1534735419866908. doi: 10.1177/1534735419866908. Integr Cancer Ther. 2019. PMID: 31416372 Free PMC article. - Dendritic Polyglycerol-Conjugated Gold Nanostars for Metabolism Inhibition and Targeted Photothermal Therapy in Breast Cancer Stem Cells.
Pan Y, Zhou S, Liu C, Ma X, Xing J, Parshad B, Li W, Wu A, Haag R. Pan Y, et al. Adv Healthc Mater. 2022 Apr;11(8):e2102272. doi: 10.1002/adhm.202102272. Epub 2022 Jan 17. Adv Healthc Mater. 2022. PMID: 34990518 Free PMC article. - Multifunctional Albumin-Stabilized Gold Nanoclusters for the Reduction of Cancer Stem Cells.
Latorre A, Latorre A, Castellanos M, Rodriguez Diaz C, Lazaro-Carrillo A, Aguado T, Lecea M, Romero-Pérez S, Calero M, Sanchez-Puelles JM, Villanueva Á, Somoza Á. Latorre A, et al. Cancers (Basel). 2019 Jul 10;11(7):969. doi: 10.3390/cancers11070969. Cancers (Basel). 2019. PMID: 31295963 Free PMC article. - The Evolving Landscape of Cancer Stem Cells and Ways to Overcome Cancer Heterogeneity.
Taniguchi H, Suzuki Y, Natori Y. Taniguchi H, et al. Cancers (Basel). 2019 Apr 14;11(4):532. doi: 10.3390/cancers11040532. Cancers (Basel). 2019. PMID: 31013960 Free PMC article. Review. - Tumor microenvironment-induced tumor cell plasticity: relationship with hypoxic stress and impact on tumor resistance.
Zaarour RF, Ribeiro M, Azzarone B, Kapoor S, Chouaib S. Zaarour RF, et al. Front Oncol. 2023 Oct 11;13:1222575. doi: 10.3389/fonc.2023.1222575. eCollection 2023. Front Oncol. 2023. PMID: 37886168 Free PMC article. Review.
References
- Ahmad A., Mondal S.K., Mukhopadhyay D., Banerjee R., Alkharfy K. M. (2016). Development of liposomal formulation for delivering anticancer drug to breast cancer stem-cell-like cells and its pharmacokinetics in an animal model. Mol. Pharm. 13, 1081–1088. 10.1021/acs.molpharmaceut.5b00900 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources