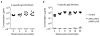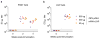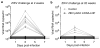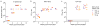Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination - PubMed (original) (raw)
. 2017 Mar 9;543(7644):248-251.
doi: 10.1038/nature21428. Epub 2017 Feb 2.
Michael J Hogan 1, Rebecca S Pelc 2, Hiromi Muramatsu 1, Hanne Andersen 3, Christina R DeMaso 2, Kimberly A Dowd 2, Laura L Sutherland 4, Richard M Scearce 4, Robert Parks 4, Wendeline Wagner 3, Alex Granados 3, Jack Greenhouse 3, Michelle Walker 3, Elinor Willis 5, Jae-Sung Yu 4, Charles E McGee 4, Gregory D Sempowski 4, Barbara L Mui 6, Ying K Tam 6, Yan-Jang Huang 7, Dana Vanlandingham 7, Veronica M Holmes 1, Harikrishnan Balachandran 8, Sujata Sahu 8, Michelle Lifton 8, Stephen Higgs 7, Scott E Hensley 5, Thomas D Madden 6, Michael J Hope 6, Katalin Karikó 9, Sampa Santra 8, Barney S Graham 10, Mark G Lewis 3, Theodore C Pierson 2, Barton F Haynes 4, Drew Weissman 1
Affiliations
- PMID: 28151488
- PMCID: PMC5344708
- DOI: 10.1038/nature21428
Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination
Norbert Pardi et al. Nature. 2017.
Abstract
Zika virus (ZIKV) has recently emerged as a pandemic associated with severe neuropathology in newborns and adults. There are no ZIKV-specific treatments or preventatives. Therefore, the development of a safe and effective vaccine is a high priority. Messenger RNA (mRNA) has emerged as a versatile and highly effective platform to deliver vaccine antigens and therapeutic proteins. Here we demonstrate that a single low-dose intradermal immunization with lipid-nanoparticle-encapsulated nucleoside-modified mRNA (mRNA-LNP) encoding the pre-membrane and envelope glycoproteins of a strain from the ZIKV outbreak in 2013 elicited potent and durable neutralizing antibody responses in mice and non-human primates. Immunization with 30 μg of nucleoside-modified ZIKV mRNA-LNP protected mice against ZIKV challenges at 2 weeks or 5 months after vaccination, and a single dose of 50 μg was sufficient to protect non-human primates against a challenge at 5 weeks after vaccination. These data demonstrate that nucleoside-modified mRNA-LNP elicits rapid and durable protective immunity and therefore represents a new and promising vaccine candidate for the global fight against ZIKV.
Figures
Extended Data Figure 1. Design and characterization of ZIKV prM-E mRNA
(a) The ZIKV mRNA encodes the signal peptide (SP) from MHC class II and prM and E glycoproteins from ZIKV H/PF/2013. (b) mRNA was transfected into 293T cells (n=3), human DC (n=3), or murine DC (n=2). E protein expression in cell lysate and supernatant was probed by Western blot, using firefly luciferase-encoding mRNA-transfected cells as a negative control. (c) ZIKV mRNA supernatant from transfected 293T cells was characterized by ultracentrifugation in the presence and absence of 0.5% Triton X-100, followed by Western blot of input (IN), pellet (P), and final supernatant (S) fractions (n=3).
Extended Data Figure 2. Nucleoside-modified ZIKV mRNA-LNP immunization elicits polyfunctional ZIKV E-specific CD4+ T cell responses
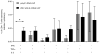
C57BL/6 mice were immunized with 30 μg of nucleoside-modified ZIKV prM-E mRNA-LNPs (n=8) or control poly(C) RNA-LNPs (n=4). At week 2, antigen-specific CD4+ T cells were detected by intracellular cytokine staining. Bar graph shows mean frequencies of combinations of cytokines produced by CD4+ T cells. Error bars indicate the SEM, and asterisk indicates a significant difference (p<0.05) by Student’s _t_-test.
Extended Data Figure 3. ZIKV E-specific IgG concentration in mice
Sera from (a) C57BL/6 mice (n=4 control; n=8 ZIKV mRNA-LNP) or (b) BALB/c mice (n=5 control; n=10 ZIKV mRNA-LNP) were assayed by ELISA, and estimates of ZIKV E-specific IgG concentrations were calculated using murine mAb NR-4747 as a standard. Points represent individual mice; horizontal lines indicate the mean. Responses in vaccine and control groups were compared at each time point by Mann-Whitney test: p<0.01 for all comparisons.
Extended Data Figure 4. Neutralizing antibody responses against ZIKV MR-766 in macaques immunized with ZIKV prM-E mRNA-LNPs
Sera from immunized macaques were evaluated for neutralization of ZIKV MR-766 using (a) the PRNT assay or (b) the RVP assay at the indicated time points. Shaded area indicates values below the limit of detection and horizontal bars indicate the mean. Immune responses in dose groups were compared by Kruskal-Wallis test: p>0.05 for all comparisons.
Extended Data Figure 5. Neutralization curve for a human anti-ZIKV neutralizing mAb
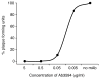
ZIKV MR-766 was neutralized by Ab3594, a human ZIKV-neutralizing monoclonal antibody, as a positive control in the PRNT assay. Shown is a representative curve (n=4). Mean EC50 = 0.026 μg/ml, SD=5.4.
Figure 1. Nucleoside-modified ZIKV mRNA-LNP immunization elicits ZIKV-specific T helper and neutralizing antibody responses
a–d, C57BL/6 mice were immunized i.d. with 30 μg of nucleoside-modified ZIKV prM-E mRNA-LNPs (n=8) or control poly(C) RNA-LNPs (n=4). (a) At week 2, splenic antigen-specific CD4+ T cells were detected by intracellular cytokine staining. The antibody response was monitored by (b) ELISA, (c) PRNT using ZIKV MR-766, and (d) RVP using ZIKV H/PF/2013. e–g, BALB/c mice were immunized similarly with ZIKV mRNA-LNPs (n=10) or poly(C) RNA-LNPs (n=5) and monitored by (e) ELISA, (f) PRNT using MR-766, and (g) RVP using H/PF/2013. Points represent individual mice; horizontal lines show the mean; shaded area indicates values below the limit of detection. The controls in d and g are from the week 8 time point. Asterisk indicates p<0.05 in unpaired t-test; antibody responses in vaccine and control groups were compared at each time point by Mann-Whitney test: p<0.01 for all comparisons.
Figure 2. A single immunization of nucleoside-modified ZIKV prM-E mRNA-LNPs provides rapid and durable protection from ZIKV challenge in mice
BALB/c mice immunized i.d. with 30 μg of ZIKV prM-E mRNA-LNPs or control poly(C) RNA-LNPs were challenged i.v. with 200 PFU ZIKV PRVABC59 at (a) 2 weeks (n=9 per group) or (b) 20 weeks (n=5 control mice; n=10 ZIKV mRNA-LNP mice) post-vaccination, and plasma viral loads were measured by qRT-PCR for ZIKV capsid RNA. ‡ symbol indicates two overlapping curves. Shaded area indicates values below the limit of detection (200 copies/ml), with undetectable curves staggered to show individual mice. Day 3 viremia in vaccine and control groups was compared by Mann-Whitney test: p<0.001 for both challenges.
Figure 3. Nucleoside-modified ZIKV mRNA-LNP immunization elicits potent ZIKV-specific neutralizing antibody responses in non-human primates
Rhesus macaques were immunized with 600 μg (n=4), 200 μg (n=3), or 50 μg (n=3) of ZIKV prM-E mRNA-LNPs, and the antibody response was quantified by (a) ELISA, (b) FRNT using ZIKV MEX I-44, and (c) RVP using ZIKV H/PF/2013. Pre-challenge (weeks 0 to 4) and unchallenged animal data are shown. Points represent individual monkeys; shaded area indicates values below the limit of detection; horizontal lines indicate the mean. Immune responses in dose groups were compared by Kruskal-Wallis test: p>0.05 for all comparisons.
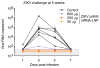
Figure 4. A single immunization of nucleoside-modified ZIKV prM-E mRNA-LNPs protects rhesus macaques from ZIKV challenge at 5 weeks post-immunization
Six unvaccinated control macaques and five vaccinated macaques that received 50 μg (n=3), 200 μg (n=1), or 600 μg (n=1) of ZIKV mRNA-LNPs at week 0 were challenged s.c. with 104 TCID50 of ZIKV PRVABC59 at week 5. Viral loads were measured in plasma by qRT-PCR for ZIKV capsid RNA. Shaded area indicates values below the limit of detection (50 copies/ml), and undetectable values were staggered to show individual animals. Day 3 and 5 viremia in vaccine and control groups was compared by Mann-Whitney test: p<0.001.
Comment in
- Zika Virus Vaccines - A Full Field and Looking for the Closers.
Thomas SJ. Thomas SJ. N Engl J Med. 2017 May 11;376(19):1883-1886. doi: 10.1056/NEJMcibr1701402. N Engl J Med. 2017. PMID: 28490001 No abstract available.
Similar articles
- Recombinant Chimpanzee Adenovirus Vaccine AdC7-M/E Protects against Zika Virus Infection and Testis Damage.
Xu K, Song Y, Dai L, Zhang Y, Lu X, Xie Y, Zhang H, Cheng T, Wang Q, Huang Q, Bi Y, Liu WJ, Liu W, Li X, Qin C, Shi Y, Yan J, Zhou D, Gao GF. Xu K, et al. J Virol. 2018 Feb 26;92(6):e01722-17. doi: 10.1128/JVI.01722-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29298885 Free PMC article. - A single-dose plasmid-launched live-attenuated Zika vaccine induces protective immunity.
Zou J, Xie X, Luo H, Shan C, Muruato AE, Weaver SC, Wang T, Shi PY. Zou J, et al. EBioMedicine. 2018 Oct;36:92-102. doi: 10.1016/j.ebiom.2018.08.056. Epub 2018 Sep 7. EBioMedicine. 2018. PMID: 30201444 Free PMC article. - A Zika virus vaccine expressing premembrane-envelope-NS1 polyprotein.
Li A, Yu J, Lu M, Ma Y, Attia Z, Shan C, Xue M, Liang X, Craig K, Makadiya N, He JJ, Jennings R, Shi PY, Peeples ME, Liu SL, Boyaka PN, Li J. Li A, et al. Nat Commun. 2018 Aug 3;9(1):3067. doi: 10.1038/s41467-018-05276-4. Nat Commun. 2018. PMID: 30076287 Free PMC article. - Zika Virus Vaccine Development: Progress in the Face of New Challenges.
Diamond MS, Ledgerwood JE, Pierson TC. Diamond MS, et al. Annu Rev Med. 2019 Jan 27;70:121-135. doi: 10.1146/annurev-med-040717-051127. Epub 2018 Nov 2. Annu Rev Med. 2019. PMID: 30388054 Review. - Potential targets for therapeutic intervention and structure based vaccine design against Zika virus.
Qadir A, Riaz M, Saeed M, Shahzad-Ul-Hussan S. Qadir A, et al. Eur J Med Chem. 2018 Aug 5;156:444-460. doi: 10.1016/j.ejmech.2018.07.014. Epub 2018 Jul 10. Eur J Med Chem. 2018. PMID: 30015077 Review.
Cited by
- A comprehensive investigation of Glycoprotein-based nucleic acid vaccines for Hantaan Virus.
Zhang J, Zhang J, Wang Y, Sun Y, Wang Y, Wang Y, Yang D, Qiao X, Liu X, Ding J, Zhang X, Zhang W, Wang Z, Hu C, Han C, Liu T, Yang S, Sun Y, Cheng L, Jiang D, Yang K. Zhang J, et al. NPJ Vaccines. 2024 Oct 23;9(1):196. doi: 10.1038/s41541-024-00991-0. NPJ Vaccines. 2024. PMID: 39443512 Free PMC article. - Designing novel multiepitope mRNA vaccine targeting Hendra virus (HeV): An integrative approach utilizing immunoinformatics, reverse vaccinology, and molecular dynamics simulation.
Mahdeen AA, Hossain I, Masum MHU, Islam S, Rabbi TMF. Mahdeen AA, et al. PLoS One. 2024 Oct 23;19(10):e0312239. doi: 10.1371/journal.pone.0312239. eCollection 2024. PLoS One. 2024. PMID: 39441880 Free PMC article. - Vax-Innate: improving therapeutic cancer vaccines by modulating T cells and the tumour microenvironment.
Baharom F, Hermans D, Delamarre L, Seder RA. Baharom F, et al. Nat Rev Immunol. 2024 Oct 21. doi: 10.1038/s41577-024-01091-9. Online ahead of print. Nat Rev Immunol. 2024. PMID: 39433884 Review. - mRNA vaccines for infectious diseases - advances, challenges and opportunities.
Pardi N, Krammer F. Pardi N, et al. Nat Rev Drug Discov. 2024 Nov;23(11):838-861. doi: 10.1038/s41573-024-01042-y. Epub 2024 Oct 4. Nat Rev Drug Discov. 2024. PMID: 39367276 Review. - mRNA vaccines: a new era in vaccine development.
Chandra S, Wilson JC, Good D, Wei MQ. Chandra S, et al. Oncol Res. 2024 Sep 18;32(10):1543-1564. doi: 10.32604/or.2024.043987. eCollection 2024. Oncol Res. 2024. PMID: 39308511 Free PMC article. Review.
References
- Weissman D. mRNA transcript therapy. Expert Rev Vaccines. 2015;14:265–281. - PubMed
- Sahin U, Kariko K, Tureci O. mRNA-based therapeutics--developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. - PubMed
- Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- UM1 AI100645/AI/NIAID NIH HHS/United States
- T32 AI007632/AI/NIAID NIH HHS/United States
- T32 AI055428/AI/NIAID NIH HHS/United States
- R01 AI090788/AI/NIAID NIH HHS/United States
- T32 AI070077/AI/NIAID NIH HHS/United States
- R01 AI124429/AI/NIAID NIH HHS/United States
- R01 AI050484/AI/NIAID NIH HHS/United States
- R01 AI041860/AI/NIAID NIH HHS/United States
- U19 AI057229/AI/NIAID NIH HHS/United States
- UC6 AI058607/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical