Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke - PubMed (original) (raw)
Review
Cerebral Vascular Disease and Neurovascular Injury in Ischemic Stroke
Xiaoming Hu et al. Circ Res. 2017.
Abstract
The consequences of cerebrovascular disease are among the leading health issues worldwide. Large and small cerebral vessel disease can trigger stroke and contribute to the vascular component of other forms of neurological dysfunction and degeneration. Both forms of vascular disease are driven by diverse risk factors, with hypertension as the leading contributor. Despite the importance of neurovascular disease and subsequent injury after ischemic events, fundamental knowledge in these areas lag behind our current understanding of neuroprotection and vascular biology in general. The goal of this review is to address select key structural and functional changes in the vasculature that promote hypoperfusion and ischemia, while also affecting the extent of injury and effectiveness of therapy. In addition, as damage to the blood-brain barrier is one of the major consequences of ischemia, we discuss cellular and molecular mechanisms underlying ischemia-induced changes in blood-brain barrier integrity and function, including alterations in endothelial cells and the contribution of pericytes, immune cells, and matrix metalloproteinases. Identification of cell types, pathways, and molecules that control vascular changes before and after ischemia may result in novel approaches to slow the progression of cerebrovascular disease and lessen both the frequency and impact of ischemic events.
Keywords: blood-brain barrier; cerebrovascular circulation; endothelium; inflammation; pericytes; risk factors.
© 2017 American Heart Association, Inc.
Figures
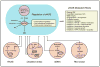
Figure 1. Risk factors and end-organ effects of vascular disease
Schematic illustration of leading risk factors for large and small vessel disease and stroke as well as key changes in vascular function, the BBB, atherosclerosis, and vascular structure. The lower portion of the figure illustrates progression vascular disease over time with major end-organ effects.
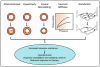
Figure 2. Mechanisms regulating function of eNOS and its impact
Activity of eNOS is increased in response to receptor- and shear stress-mediated effects. Activation of transient receptor potential V4 channels (TRPV4) is involved for some stimuli. Enzyme activity is dependent on L-arginine, calcium (Ca2+), calmodulin (CaM), and tetrahydrobiopterin (BH4) and is inhibited by caveolin-1. eNOS-mediated effects in healthy endothelium is show in the upper right. The bottom of the figure highlights major mechanisms that contribute to endothelial dysfunction, including the RAAS, oxidative stress, asymmetric dimethylarginine (ADMA), and Rho kinase. See text for additional details. Aldo, aldosterone; AT1R, AT1 receptor; Mito, mitochondria; ONOO−, peroxynitrite; O2−, superoxide; GPx, glutathione peroxidase; DDAH, dimethylarginine dimethylaminohydrolase (DDAH); ET1R, endothelin-1 receptor.
Figure 3. Changes in vascular structure and mechanics
Major structural and mechanical changes in the vasculature (shown in cross-section) that collectively reduce the vascular lumen, affect vasodilator responses, and limit vasodilator reserve (increase minimal vascular resistance). Physiological consequences of these changes are listed on the bottom.
Figure 4. The structural alterations in endothelial cells are critical for BBB opening early after ischemic stroke
A. The opening of paracellular pathways: cytoskeletal rearrangements and related signaling in endothelial cells. B. The transcellular pathway of BBB leakage. ADF: actin-depolymerizing factor; CaM: calmodulin; LIMK: LIM kinase; MLC: myosin light chain; MLCK: MLC kinase; MLCP: MLC phosphatase; MMP: matrix metallopeptidase; ROCK: Rho kinase TESK1: testicular protein kinase 1.
Figure 5. Pericytes play multifaceted roles in ischemia and reperfusion
Pericytes display both beneficial and detrimental functions during ischemia and reperfusion phases, and contribute significantly to the BBB damage and repair. 1) Pericyte contraction and dilation regulate cerebral blood flow in the ischemic and peri-lesion areas. 2) Pericyte protects other NVU components through releasing protective/trophic factors such as nerve growth factor (NGF), neurotrophin-3 (NT-3), vascular endothelial growth factor (VEGF), angiopoietin (Ang-1) and glial cell line-derived neurotrophic factor (GDNF). 3) Phagocytotic pericytes help to eliminate dead or injured tissue in the ischemic core, which in turn mitigates local inflammation and reduce secondary tissue damage. 4) Pericyte-endothelial cell interaction promotes angiogenesis after stroke. 5) Pericytes have the potential to serve as an origin of NVU components during tissue repair after ischemic stroke.
Similar articles
- Cerebrovascular Disease: Consequences of Obesity-Induced Endothelial Dysfunction.
Letra L, Sena C. Letra L, et al. Adv Neurobiol. 2017;19:163-189. doi: 10.1007/978-3-319-63260-5_7. Adv Neurobiol. 2017. PMID: 28933065 Review. - Non-coding RNAs in cerebral endothelial pathophysiology: emerging roles in stroke.
Yin KJ, Hamblin M, Chen YE. Yin KJ, et al. Neurochem Int. 2014 Nov;77:9-16. doi: 10.1016/j.neuint.2014.03.013. Epub 2014 Apr 3. Neurochem Int. 2014. PMID: 24704794 Free PMC article. Review. - Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice.
McColl BW, Rothwell NJ, Allan SM. McColl BW, et al. J Neurosci. 2008 Sep 17;28(38):9451-62. doi: 10.1523/JNEUROSCI.2674-08.2008. J Neurosci. 2008. PMID: 18799677 Free PMC article. - Neurovascular mechanisms and blood-brain barrier disorder in Alzheimer's disease.
Bell RD, Zlokovic BV. Bell RD, et al. Acta Neuropathol. 2009 Jul;118(1):103-13. doi: 10.1007/s00401-009-0522-3. Epub 2009 Mar 25. Acta Neuropathol. 2009. PMID: 19319544 Free PMC article. Review. - Pericytes in Brain Injury and Repair After Ischemic Stroke.
Cai W, Liu H, Zhao J, Chen LY, Chen J, Lu Z, Hu X. Cai W, et al. Transl Stroke Res. 2017 Apr;8(2):107-121. doi: 10.1007/s12975-016-0504-4. Epub 2016 Nov 12. Transl Stroke Res. 2017. PMID: 27837475 Free PMC article. Review.
Cited by
- Xingnaojing injection alleviates cerebral ischemia/reperfusion injury through regulating endoplasmic reticulum stress in Vivo and in Vitro.
Dong X, Li C, Yao Y, Liu F, Jiang P, Gao Y. Dong X, et al. Heliyon. 2024 Jan 24;10(3):e25267. doi: 10.1016/j.heliyon.2024.e25267. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38327400 Free PMC article. - Cerebral Blood Flow Response to Simulated Hypovolemia in Essential Hypertension: A Magnetic Resonance Imaging Study.
Neumann S, Burchell AE, Rodrigues JCL, Lawton CB, Burden D, Underhill M, Kobetić MD, Adams ZH, Brooks JCW, Nightingale AK, Paton JFR, Hamilton MCK, Hart EC. Neumann S, et al. Hypertension. 2019 Dec;74(6):1391-1398. doi: 10.1161/HYPERTENSIONAHA.119.13229. Epub 2019 Oct 28. Hypertension. 2019. PMID: 31656098 Free PMC article. - Platelets, Thromboinflammation and Neurovascular Disease.
Sun Y, Langer HF. Sun Y, et al. Front Immunol. 2022 Mar 4;13:843404. doi: 10.3389/fimmu.2022.843404. eCollection 2022. Front Immunol. 2022. PMID: 35309326 Free PMC article. Review. - Reappraisal of the Concept of Accelerated Aging in Neurodegeneration and Beyond.
Statsenko Y, Kuznetsov NV, Morozova D, Liaonchyk K, Simiyu GL, Smetanina D, Kashapov A, Meribout S, Gorkom KN, Hamoudi R, Ismail F, Ansari SA, Emerald BS, Ljubisavljevic M. Statsenko Y, et al. Cells. 2023 Oct 14;12(20):2451. doi: 10.3390/cells12202451. Cells. 2023. PMID: 37887295 Free PMC article. Review. - Cellular and molecular mechanisms in vascular repair after traumatic brain injury: a narrative review.
Zhao ZA, Yan L, Wen J, Satyanarayanan SK, Yu F, Lu J, Liu YU, Su H. Zhao ZA, et al. Burns Trauma. 2023 Sep 4;11:tkad033. doi: 10.1093/burnst/tkad033. eCollection 2023. Burns Trauma. 2023. PMID: 37675267 Free PMC article. Review.
References
- Writing Group M. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. Heart disease and stroke statistics-2016 update: A report from the American Heart Association. Circulation. 2016;133:e38–60. - PubMed
- Korczyn AD. Vascular Parkinsonism - characteristics, pathogenesis and treatment. Nature Rev Neurol. 2015;11:319–326. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 NS092618/NS/NINDS NIH HHS/United States
- R01 NS089534/NS/NINDS NIH HHS/United States
- I01 BX002495/BX/BLRD VA/United States
- R01 HL113863/HL/NHLBI NIH HHS/United States
- R01 NS091175/NS/NINDS NIH HHS/United States
- R01 NS045048/NS/NINDS NIH HHS/United States
- R01 NS096465/NS/NINDS NIH HHS/United States
- R01 NS095671/NS/NINDS NIH HHS/United States
- P01 HL062984/HL/NHLBI NIH HHS/United States
- I01 BX001399/BX/BLRD VA/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical