Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction - PubMed (original) (raw)
. 2017 Jan 14;38(3):201-211.
doi: 10.1093/eurheartj/ehw240.
James Dawkins 1, Jackelyn Valle 1, Eli Simsolo 1, Geoffrey de Couto 1, Ryan Middleton 1, Eleni Tseliou 1, Daniel Luthringer 1, Michelle Kreke 1 3, Rachel R Smith 3, Linda Marbán 1 3, Bijan Ghaleh 2, Eduardo Marbán 1
Affiliations
- PMID: 28158410
- PMCID: PMC5837390
- DOI: 10.1093/eurheartj/ehw240
Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction
Romain Gallet et al. Eur Heart J. 2017.
Abstract
Aims: Naturally secreted nanovesicles known as exosomes are required for the regenerative effects of cardiosphere-derived cells (CDCs), and exosomes mimic the benefits of CDCs in rodents. Nevertheless, exosomes have not been studied in a translationally realistic large-animal model. We sought to optimize delivery and assess the efficacy of CDC-secreted exosomes in pig models of acute (AMI) and convalescent myocardial infarction (CMI).
Methods and results: In AMI, pigs received human CDC exosomes (or vehicle) by intracoronary (IC) or open-chest intramyocardial (IM) delivery 30 min after reperfusion. No-reflow area and infarct size (IS) were assessed histologically at 48 h. Intracoronary exosomes were ineffective, but IM exosomes decreased IS from 80 ± 5% to 61 ± 12% (P= 0.001) and preserved left ventricular ejection fraction (LVEF). In a randomized placebo-controlled study of CMI, pigs 4 weeks post-myocardial infarction (MI) underwent percutaneous IM delivery of vehicle (n = 6) or CDC exosomes (n = 6). Magnetic resonance imaging (MRI) performed before and 1 month after treatment revealed that exosomes (but not vehicle) preserved LV volumes and LVEF (−0.1 ± 2.2% vs. −5.4 ± 3.6%, P= 0.01) while decreasing scar size. Histologically, exosomes decreased LV collagen content and cardiomyocyte hypertrophy while increasing vessel density.
Conclusion: Cardiosphere-derived cell exosomes delivered IM decrease scarring, halt adverse remodelling and improve LVEF in porcine AMI and CMI. While conceptually attractive as cell-free therapeutic agents for myocardial infarction, exosomes have the disadvantage that IM delivery is necessary.
Figures
Figure 1
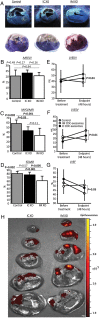
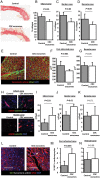
Acute myocardial infarction study: infarct size, micro-vascular occlusion, and retention. (A) Representative images of heart sections under UV-light (top panels) and after TTC staining (bottom panels) in control, intracoronary exosomes, and intramyocardial-treated pigs. Under UV light, micro-vascular obstruction appears dark and area-at-risk fluorescent; after TTC staining, scar appears white and area-at-risk red; non-ischaemic myocardium appears purple. Pooled data show that area-at-risk/left ventricular is similar in the three groups (B), both intracoronary and intramyocardial exosomes decrease micro-vascular obstruction compared with control (C), and intramyocardial exosomes but not intracoronary exosomes decrease scar size compared with control (D). Intramyocardial exosomes (but not intracoronary exosomes) preserve left ventricular end-diastolic (E) and end-systolic volume (F) and left ventricular ejection fraction. (H) Bioluminescence tracking in a heart infused via the intracoronary route with far-red labelled exosomes (left) and another heart that had been injected intramyocardially with exosomes (right); signal intensity is much higher after intramyocardial injection.
Figure 2
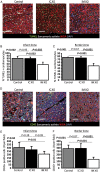
Acute myocardial infarction study: apoptosis and inflammation. (A) Representative images of TUNEL staining for apoptosis quantification in a control, an intracoronary exosome, and an intramyocardial exosome-treated pig (border zone). Pooled data show that cardiomyocyte apoptosis in the infarcted (B) and border (C) area is reduced by intramyocardial exosomes but not by intracoronary exosomes compared with control. (D) Representative images of CD45 staining for quantification of leukocyte infiltration in a control, an intracoronary exosome and an intramyocardial exosome-treated pig (border zone). Pooled data show that leukocyte infiltration in the infarcted (E) and border (F) area is reduced by intramyocardial exosomes but not by intracoronary exosomes compared with control. Scale bars = 50 μm.
Figure 3
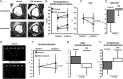
Randomized pre-clinical study in convalescent myocardial infarction: structure and function. (A) Representative short-axis end-diastolic (top) and end-systolic (bottom) magnetic resonance images at endpoint in control (left) and cardiosphere-derived cell exosome-treated (right) pigs. (B) Pooled data show that left ventricular end-diastolic and end-systolic volumes are similar in the two groups at baseline but end-systolic volume increases only in the vehicle group at endpoint (not in the cardiosphere-derived cell exosome-treated animals). Left ventricular ejection fraction is similar in the two groups at baseline but is higher in cardiosphere-derived cell exosome-treated pigs at endpoint (C), and the decrease in left ventricular ejection fraction is greater in the control-treated animals (D). (E) Representative images of global circumferential strain at endpoint in a cardiosphere-derived cell exosome and a control-treated pig. Pooled data show that circumferential strain is similar in the two groups at baseline (F) but improves (decrease of value shown in [G], absolute improvement in [H]) in the cardiosphere-derived cell exosome-treated pigs while it decreases in the control pigs at endpoint. * P< 0.05 vs. baseline (intra-group paired analysis).
Figure 4
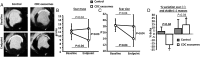
Randomized pre-clinical study in convalescent myocardial infarction: scar mass and viable mass. (A) Representative MR images of short-axis late-gadolinium enhancement at baseline and endpoint in a control and a cardiosphere-derived cell exosome-treated pigs. Pooled data show that scar mass (B) and scar size (C) are similar at baseline in the two groups but decrease at endpoint in cardiosphere-derived cell exosome-treated pigs compared with control. Consequently, viable mass increases only in cardiosphere-derived cell exosome-treated pigs (D). * P< 0.05 vs. baseline (intra-group paired analysis).
Figure 5
Randomized pre-clinical study in convalescent myocardial infarction: histological quantification of infarction. (A) Representative images of TTC stained heart from a control (left) and a cardiosphere-derived cell exosome-treated heart (right). Pooled data show that scar mass (B), scar size (C), and transmurality (D) at endpoint are lower in the cardiosphere-derived cell exosome-treated animals compared with control while viable mass tends to be higher (E).
Figure 6
Randomized pre-clinical study in convalescent myocardial infarction: fibrosis, vascular density, and cardiomyocyte proliferation. (A) Representative images of infarcted area stained with picrosirius red in a control and a cardiosphere-derived cell exosome-treated pig. Pooled data show that collagen content of the infarcted area (B), the border zone (C), and the remote area (D) at endpoint is lower in cardiosphere-derived cell exosomes-treated pigs compared with control. (E) Representative images of cross-sectional area in a control and a cardiosphere-derived cell exosome-treated pig. Pooled data show that cardiomyocytes of cardiosphere-derived cell exosome-treated pigs are smaller in the peri-infarcted area (F) but not in the remote area (G). (H) Representative images of arteriole density in a control and a cardiosphere-derived cell exosome-treated pig (infarct and border zone). Pooled data show that, compared with control, cardiosphere-derived cell exosomes increase vascular density at endpoint in the infarct (I) and border area (J) but not in the remote area (K). (L) Representative images of Ki67 staining in the peri-infarct area in a control and a cardiosphere-derived cell exosome-treated pig (arrow shows a Ki67 positive cardiomyocyte). Pooled data show that proliferation of cardiomyocytes in the peri-infarct area (M) but not in the remote area (N) is higher in cardiosphere-derived cell exosome-treated pigs than in control. Scale bars = 50 μm.
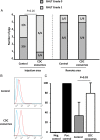
Figure 7
Randomized pre-clinical study in convalescent myocardial infarction: immunology. (A) Cellular reactions in the injection area and remote area are not different between control and exosome-treated animals. (B) Representative data of allo-antibodies quantification by flow cytometry in a control and an exosome-treated animal (blue is negative control, red is the animal serum). Pooled data show that allo-antibodies were detected in both control and exosome-treated animals, although at higher levels in the latter (C).
Comment in
- Cardiac cell-derived exosomes: changing face of regenerative biology.
Kishore R, Khan M. Kishore R, et al. Eur Heart J. 2017 Jan 14;38(3):212-215. doi: 10.1093/eurheartj/ehw324. Eur Heart J. 2017. PMID: 28158461 Free PMC article. No abstract available.
Similar articles
- Durable Benefits of Cellular Postconditioning: Long-Term Effects of Allogeneic Cardiosphere-Derived Cells Infused After Reperfusion in Pigs with Acute Myocardial Infarction.
Kanazawa H, Tseliou E, Dawkins JF, De Couto G, Gallet R, Malliaras K, Yee K, Kreke M, Valle I, Smith RR, Middleton RC, Ho CS, Dharmakumar R, Li D, Makkar RR, Fukuda K, Marbán L, Marbán E. Kanazawa H, et al. J Am Heart Assoc. 2016 Feb 8;5(2):e002796. doi: 10.1161/JAHA.115.002796. J Am Heart Assoc. 2016. PMID: 26857066 Free PMC article. - Nonocclusive multivessel intracoronary infusion of allogeneic cardiosphere-derived cells early after reperfusion prevents remote zone myocyte loss and improves global left ventricular function in swine with myocardial infarction.
Suzuki G, Weil BR, Young RF, Fallavollita JA, Canty JM Jr. Suzuki G, et al. Am J Physiol Heart Circ Physiol. 2019 Aug 1;317(2):H345-H356. doi: 10.1152/ajpheart.00124.2019. Epub 2019 May 24. Am J Physiol Heart Circ Physiol. 2019. PMID: 31125261 Free PMC article. - Intracoronary delivery of self-assembling heart-derived microtissues (cardiospheres) for prevention of adverse remodeling in a pig model of convalescent myocardial infarction.
Gallet R, Tseliou E, Dawkins J, Middleton R, Valle J, Angert D, Reich H, Luthringer D, Kreke M, Smith R, Marbán L, Marbán E. Gallet R, et al. Circ Cardiovasc Interv. 2015 May;8(5):10.1161/CIRCINTERVENTIONS.115.002391 e002391. doi: 10.1161/CIRCINTERVENTIONS.115.002391. Circ Cardiovasc Interv. 2015. PMID: 25953823 Free PMC article. - Exosomes Induce Crosstalk Between Multiple Types of Cells and Cardiac Fibroblasts: Therapeutic Potential for Remodeling After Myocardial Infarction.
Feng Y, Wang Y, Li L, Yang Y, Tan X, Chen T. Feng Y, et al. Int J Nanomedicine. 2024 Oct 19;19:10605-10621. doi: 10.2147/IJN.S476995. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39445157 Free PMC article. Review. - Regulatory T cells as a therapeutic target in acute myocardial infarction.
Wu Q, Wu M, Zhang K, Sun R, Li H, Tong J, Guo Y. Wu Q, et al. Mol Immunol. 2024 Aug;172:17-22. doi: 10.1016/j.molimm.2024.06.003. Epub 2024 Jun 11. Mol Immunol. 2024. PMID: 38865800 Review.
Cited by
- Exosomes and Exosomal Cargos: A Promising World for Ventricular Remodeling Following Myocardial Infarction.
Fang J, Zhang Y, Chen D, Zheng Y, Jiang J. Fang J, et al. Int J Nanomedicine. 2022 Oct 4;17:4699-4719. doi: 10.2147/IJN.S377479. eCollection 2022. Int J Nanomedicine. 2022. PMID: 36217495 Free PMC article. Review. - Exosomes: Potential Therapies for Disease via Regulating TLRs.
Guo HY, Cheng AC, Wang MS, Yin ZQ, Jia RY. Guo HY, et al. Mediators Inflamm. 2020 May 27;2020:2319616. doi: 10.1155/2020/2319616. eCollection 2020. Mediators Inflamm. 2020. PMID: 32565722 Free PMC article. Review. - A commentary on: TDO2-augmented fibroblasts secrete EVs enriched in immunomodulatory Y-derived small RNA.
Sastrawidjaya C, Nguyen PHD. Sastrawidjaya C, et al. J Extracell Biol. 2023 Aug 18;2(8):e99. doi: 10.1002/jex2.99. eCollection 2023 Aug. J Extracell Biol. 2023. PMID: 38939510 Free PMC article. No abstract available. - Extracellular vesicles in cardiovascular diseases.
Fu S, Zhang Y, Li Y, Luo L, Zhao Y, Yao Y. Fu S, et al. Cell Death Discov. 2020 Jul 30;6:68. doi: 10.1038/s41420-020-00305-y. eCollection 2020. Cell Death Discov. 2020. PMID: 32821437 Free PMC article. Review. - Progenitor Cells Derived from Drain Waste Product of Open-Heart Surgery in Children.
Wong TW, Kan CD, Chiu WT, Fok KL, Ruan YC, Jiang X, Chen J, Kao CC, Chen IY, Lin HC, Chou CH, Lin CW, Yu CK, Tsao S, Lee YP, Chan HC, Wang JN. Wong TW, et al. J Clin Med. 2019 Jul 12;8(7):1028. doi: 10.3390/jcm8071028. J Clin Med. 2019. PMID: 31336927 Free PMC article.
References
- World Health Organisation. Global atlas on cardiovascular disease prevention and control. 2011.
- Allogeneic Heart Stem Cells to Achieve Myocardial Regeneration (ALLSTAR) (NCT01458405) http://clinicaltrials.gov/ct2/show/NCT01458405?term=allstar&rank=1 (28 May 2016, date last accessed). - PMC - PubMed
- Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 2009;120:1075–1083, 7 p following 1083. - PMC - PubMed
- Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LEJ, Berman D, Czer LSC, Marbán L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, Marbán E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. The Lancet 2012;379:895–904. - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical