Molecular Architecture of the Major Membrane Ring Component of the Nuclear Pore Complex - PubMed (original) (raw)
. 2017 Mar 7;25(3):434-445.
doi: 10.1016/j.str.2017.01.006. Epub 2017 Feb 2.
Seung Joong Kim 2, Parthasarathy Sampathkumar 3, Kaushik Dutta 4, Sean M Cahill 3, Ilan E Chemmama 2, Rosemary Williams 5, Jeffrey B Bonanno 3, William J Rice 6, David L Stokes 7, David Cowburn 3, Steven C Almo 3, Andrej Sali 8, Michael P Rout 9, Javier Fernandez-Martinez 10
Affiliations
- PMID: 28162953
- PMCID: PMC5342941
- DOI: 10.1016/j.str.2017.01.006
Molecular Architecture of the Major Membrane Ring Component of the Nuclear Pore Complex
Paula Upla et al. Structure. 2017.
Abstract
The membrane ring that equatorially circumscribes the nuclear pore complex (NPC) in the perinuclear lumen of the nuclear envelope is composed largely of Pom152 in yeast and its ortholog Nup210 (or Gp210) in vertebrates. Here, we have used a combination of negative-stain electron microscopy, nuclear magnetic resonance, and small-angle X-ray scattering methods to determine an integrative structure of the ∼120 kDa luminal domain of Pom152. Our structural analysis reveals that the luminal domain is formed by a flexible string-of-pearls arrangement of nine repetitive cadherin-like Ig-like domains, indicating an evolutionary connection between NPCs and the cell adhesion machinery. The 16 copies of Pom152 known to be present in the yeast NPC are long enough to form the observed membrane ring, suggesting how interactions between Pom152 molecules help establish and maintain the NPC architecture.
Keywords: Gp210; NMR; Nup210; Pom152; SAXS; cadherin; electron microscopy; integrative structure determination; nuclear pore complex; nucleoporin.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Competing Financial Interests
The authors declare no competing financial interest.
Figures
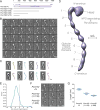
Figure 1. Negative stain EM analysis shows that Pom152 has an extended, string-of-pearls shaped luminal domain
(A) Domain organization of Pom152FL and four truncations drawn to scale. Pom152FL exhibits a three-domain organization with the domain inside the NPC (NPC), the transmembrane segment (TM), and the domain inside the nuclear envelope lumen (luminal domain). The numbers indicate amino acid residue positions. Horizontal grey lines for the truncations represent the number of amino acid residues in each segment. (B) 35 representative negative-stain EM class averages of Pom152FL. The number of particles in each class is shown. Bar, 10 nm. (C) Representative random conical tilt 3D maps (right) are aligned to each of the corresponding class averages (left). The number of particles in each class is shown. Bars, 10 nm. (D) Negative-stain EM density map of Pom152FL. The nine globular domains in C-terminal lumen (1 to 9) and the N-terminal head region containing the NPC-associating and TM domains are indicated. Bar, 50 Å. (E) The average inter-domain angle for the last 7 repetitive regions was estimated in 37 representative Pom152FL class averages using the ImageJ angle tool. One angle was measured between domains 3 and 9, its difference from 180° was determined and the resulting value divided between the seven globular domains involved in the estimation. Distribution of the resulting inter-domain angles is shown in a Kernel density plot with a peak of −4.1° indicating a small negative curvature for the particles. (F) Representative negative-stain EM class averages of three assigned views of Pom152FL, Pom1521–1135, and Pom1521–936. The number of particles in each class is shown. Bar, 10 nm. (G) End-to-end distances of Pom152FL, Pom1521–1135, and Pom1521–936 in samples of 30, 28, and 33 class averages, respectively. The lines on the data points indicate the mean and SD: 38.7 ±1.5 nm, 28.9 ±1.6 nm and 24.4 ±1.0 nm for the samples, respectively. See also Figure S1 and Table S2.
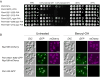
Figure 2. Functional analysis of truncations affecting the Pom152 luminal domain
(A) Growth phenotypes of Pom152FL tagged with Protein A or prescission protease cleavage site (PPX)-Protein A and related truncation mutants (see Figure 1A). Serial 10-fold dilutions of cells were spotted on YPD plates in the absence or presence of 0.3% and 0.4% benzyl-OH at 30°C or on YPD plates an d grown at the indicated temperatures for 1–3 days. (B) Subcellular localization of benzyl-OH treated Pom152-GFP constructs. Panels show the localization of the indicated genomically tagged Pom152-GFP or Nup188-mCherry (used as NPC localization control) constructs as determined by fluorescence microscopy. Cells were grown on liquid yeast minimal media supplemented with 2% glucose at 30° C (untreated) or with an additional 0.1% benzyl-OH for 3 h at 30° C (benzyl-OH). Differential interference contrast (DIC). Bar, 5 microns. See also Figure 1A and Table S2.
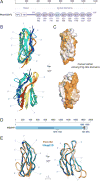
Figure 3. NMR structure determination of Pom152718–820 reveals a conserved Ig-like fold domain
(A) Superimposition of 20 lowest-energy structures of Pom152718–820 that satisfy the NMR restraints best. The two β-sheets are made of the ABE (blue) and C′CFGG′A′ β-strands (cyan). (B) Topology of the Pom152718–820 fold. β-strands are represented as thick arrows and the linkers connecting them as lines. Each strand is named following the standard Ig-like domain annotation (Halaby et al., 1999). Scale bar, 5 Å. (C and D) Two views of the best-scoring structure, showing the arrangement of β-strands and features labeled as in panel B. (E and F) Sequence conservation of Pom152718–820 among 37 fungal homologs (Figure S6), plotted on the surface of the Pom152718–820 structure; low conservation in white, high conservation in red. (G and H) Electrostatic potential on the surface of the Pom152718–820 structure, calculated using the PyMOL tool APBS; negative (−2 kT/e) and positive (+2 kT/e) potentials are shown in red and blue, respectively. See also Figure S2 and Table 1.
Figure 4. Comparative models of 8 Ig-like domains and comparison to an Ig-like domain in human Nup210
(A) Domain organization of Pom152FL and its domains drawn to scale. The head region contains the domain that faces the NPC inner ring (NPC) and the transmembrane segment (TM), followed by the lumenal domain composed of nine Ig-like repeats. The amino acid boundaries for each Ig-like repeat are indicated below them. The repeat analyzed by NMR (Ig-4, Pom152718–820) is highlighted in light blue. (B) Comparative models of the remaining 8 Ig-like domains were built using the Pom152718–820 NMR structure as the template, followed by superposing them on the Pom152718–820 structure. The 8 domains share statistically significant sequence alignments to Pom152718–820 (E-values ranging from 3.3×10−49 to 6.8×10−39). (C) Sequence conservation among the 9 Ig-like domains was mapped on the structure of Pom152718–820 using ConSurf (Ashkenazy et al., 2016). Low conservation, white; high conservation, orange. (D) Domain organization of human Nup210. See legend of Figure 1A. (E) Superposition of comparative models for yeast Pom152718–820 (orange) and human Nup2101079–1152 (blue). See also Figure S3.
Figure 5. 4-stage scheme for integrative structure determination of Pom152FL
The integrative structure determination of Pom152FL proceeds through 4 stages: (1) gathering data, (2) representing and translating data into spatial restraints, (3) conformational sampling to produce an ensemble of structures that satisfies the restraints, and (4) analyzing, assessing, and validating the ensemble structures. The modeling protocol (i.e., stages 2, 3, and 4) was scripted using the Python Modeling Interface (PMI), version 4d97507, a library for modeling macromolecular complexes based on our open-source Integrative Modeling Platform (IMP) package, version 2.6 (
http://integrativemodeling.org
) (Russel et al., 2012).
Figure 6. Integrative structure of Pom152FL based on NMR spectroscopy, negative-stain EM, and SAXS
(A) (left) The localization probability density map computed from 364 superposed structures that satisfy the input spatial restraints shows the location of each of the 9 Ig-like domains (ranging from blue to red). The negative-stain EM density map is superposed in grey. A representative molecular model of Pom152LD (ribbon plot) was obtained by adjusting the relative orientations of adjacent Ig-like domains to resemble those in known cadherin structures (PDB codes 1L3W, 1EPF, 1NCI, 4ZI9, and 5K8R) (Boggon et al., 2002; Kasper et al., 2000; Nicoludis et al., 2015; Nicoludis et al., 2016; Shapiro et al., 1995). (right) Validation of the integrative structure of Pom152LD by SAXS data for five Pom152 segments, spanning residues 718–820 (Ig-4), 718–920 (Ig-4,5), 603–820 (Ig-3,4), 919–1020 (Ig-6), and 718–1148 (Ig-4,5,6,7). The shapes of these segments in our integrative structure match the envelopes (ab initio shapes) computed from the corresponding SAXS profiles. (B) Fit of 16 copies of Pom152LD into the yeast NPC map (Alber et al., 2007b). A good fit positions two copies of the extended Pom152LD molecule in an anti-parallel fashion on top of each other, forming a homodimer; we only show the potential arrangement of the antiparallel homodimer, which is implied by the C2-symmetry of the NPC (Alber et al., 2007b; Kosinski et al., 2016; Lin et al., 2016). See also Figures 5, S4–S7 and Table S1.
Similar articles
- Characterization of nuclear pore complex targeting domains in Pom152 in Saccharomyces cerevisiae.
Brown JT, Haraczy AJ, Wilhelm CM, Belanger KD. Brown JT, et al. Biol Open. 2021 Oct 15;10(10):bio057661. doi: 10.1242/bio.057661. Epub 2021 Oct 20. Biol Open. 2021. PMID: 34557894 Free PMC article. - Structure, dynamics, evolution, and function of a major scaffold component in the nuclear pore complex.
Sampathkumar P, Kim SJ, Upla P, Rice WJ, Phillips J, Timney BL, Pieper U, Bonanno JB, Fernandez-Martinez J, Hakhverdyan Z, Ketaren NE, Matsui T, Weiss TM, Stokes DL, Sauder JM, Burley SK, Sali A, Rout MP, Almo SC. Sampathkumar P, et al. Structure. 2013 Apr 2;21(4):560-71. doi: 10.1016/j.str.2013.02.005. Epub 2013 Mar 14. Structure. 2013. PMID: 23499021 Free PMC article. - Comprehensive structure and functional adaptations of the yeast nuclear pore complex.
Akey CW, Singh D, Ouch C, Echeverria I, Nudelman I, Varberg JM, Yu Z, Fang F, Shi Y, Wang J, Salzberg D, Song K, Xu C, Gumbart JC, Suslov S, Unruh J, Jaspersen SL, Chait BT, Sali A, Fernandez-Martinez J, Ludtke SJ, Villa E, Rout MP. Akey CW, et al. Cell. 2022 Jan 20;185(2):361-378.e25. doi: 10.1016/j.cell.2021.12.015. Epub 2022 Jan 3. Cell. 2022. PMID: 34982960 Free PMC article. - [Nuclear pores: from yeast to higher eukaryotes].
Doye V. Doye V. J Soc Biol. 2002;196(4):349-54. J Soc Biol. 2002. PMID: 12645306 Review. French. - The structure of the nuclear pore complex.
Hoelz A, Debler EW, Blobel G. Hoelz A, et al. Annu Rev Biochem. 2011;80:613-43. doi: 10.1146/annurev-biochem-060109-151030. Annu Rev Biochem. 2011. PMID: 21495847 Review.
Cited by
- From integrative structural biology to cell biology.
Sali A. Sali A. J Biol Chem. 2021 Jan-Jun;296:100743. doi: 10.1016/j.jbc.2021.100743. Epub 2021 May 4. J Biol Chem. 2021. PMID: 33957123 Free PMC article. Review. - Lipid Droplets Are a Physiological Nucleoporin Reservoir.
Kumanski S, Viart BT, Kossida S, Moriel-Carretero M. Kumanski S, et al. Cells. 2021 Feb 22;10(2):472. doi: 10.3390/cells10020472. Cells. 2021. PMID: 33671805 Free PMC article. - Function of Nuclear Pore Complexes in Regulation of Plant Defense Signaling.
Wu X, Han J, Guo C. Wu X, et al. Int J Mol Sci. 2022 Mar 11;23(6):3031. doi: 10.3390/ijms23063031. Int J Mol Sci. 2022. PMID: 35328452 Free PMC article. Review. - Expression of TorsinA in a heterologous yeast system reveals interactions with lumenal domains of LINC and nuclear pore complex components.
Chalfant M, Barber KW, Borah S, Thaller D, Lusk CP. Chalfant M, et al. Mol Biol Cell. 2019 Mar 1;30(5):530-541. doi: 10.1091/mbc.E18-09-0585. Epub 2019 Jan 9. Mol Biol Cell. 2019. PMID: 30625036 Free PMC article. - Distinct domains in Ndc1 mediate its interaction with the Nup84 complex and the nuclear membrane.
Amm I, Weberruss M, Hellwig A, Schwarz J, Tatarek-Nossol M, Lüchtenborg C, Kallas M, Brügger B, Hurt E, Antonin W. Amm I, et al. J Cell Biol. 2023 Jun 5;222(6):e202210059. doi: 10.1083/jcb.202210059. Epub 2023 May 8. J Cell Biol. 2023. PMID: 37154843 Free PMC article.
References
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. Determining the architectures of macromolecular assemblies. Nature. 2007a;450:683–694. - PubMed
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, et al. The molecular architecture of the nuclear pore complex. Nature. 2007b;450:695–701. - PubMed
- Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. - PubMed
MeSH terms
Substances
Grants and funding
- T32 GM008284/GM/NIGMS NIH HHS/United States
- R01 GM083960/GM/NIGMS NIH HHS/United States
- P41 GM103314/GM/NIGMS NIH HHS/United States
- T32 EB009383/EB/NIBIB NIH HHS/United States
- S10 OD016305/OD/NIH HHS/United States
- U54 RR022220/RR/NCRR NIH HHS/United States
- R01 GM112108/GM/NIGMS NIH HHS/United States
- R01 GM117212/GM/NIGMS NIH HHS/United States
- U54 GM094662/GM/NIGMS NIH HHS/United States
- P41 GM109824/GM/NIGMS NIH HHS/United States
- S10 OD021596/OD/NIH HHS/United States
- U01 GM098256/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases