Novel neuro-audiological findings and further evidence for TWNK involvement in Perrault syndrome - PubMed (original) (raw)
doi: 10.1186/s12967-017-1129-4.
Dominika Oziębło 2, Agnieszka Pollak 2, Iwona Stępniak 2, Michal Lazniewski 3, Urszula Lechowicz 2, Krzysztof Kochanek 4, Mariusz Furmanek 5, Grażyna Tacikowska 6, Dariusz Plewczynski 3, Tomasz Wolak 5, Rafał Płoski 7, Henryk Skarżyński 8
Affiliations
- PMID: 28178980
- PMCID: PMC5299684
- DOI: 10.1186/s12967-017-1129-4
Novel neuro-audiological findings and further evidence for TWNK involvement in Perrault syndrome
Monika Ołdak et al. J Transl Med. 2017.
Abstract
Background: Hearing loss and ovarian dysfunction are key features of Perrault syndrome (PRLTS) but the clinical and pathophysiological features of hearing impairment in PRLTS individuals have not been addressed. Mutations in one of five different genes HSD17B4, HARS2, LARS2, CLPP or TWNK (previous symbol C10orf2) cause the autosomal recessive disorder but they are found only in about half of the patients.
Methods: We report on two siblings with a clinical picture resembling a severe, neurological type of PRLTS. For an exhaustive characterisation of the phenotype neuroimaging with volumetric measurements and objective measures of cochlear hair cell and auditory nerve function (otoacustic emissions and auditory brainstem responses) were used. Whole exome sequencing was applied to identify the genetic cause of the disorder. Co-segregation of the detected mutations with the phenotype was confirmed by Sanger sequencing. In silico analysis including 3D protein structure modelling was used to predict the deleterious effects of the detected variants on protein function.
Results: We found two rare biallelic mutations in TWNK, encoding Twinkle, an essential mitochondrial helicase. Mutation c.1196A>G (p.Asn399Ser) recurred for the first time in a patient with PRLTS and the second mutation c.1802G>A (p.Arg601Gln) was novel for the disorder. In both patients neuroimaging studies showed diminished cervical enlargement of the spinal cord and for the first time in PRLTS partial atrophy of the vestibulocochlear nerves and decreased grey and increased white matter volumes of the cerebellum. Morphological changes in the auditory nerves, their desynchronized activity and partial cochlear dysfunction underlay the complex mechanism of hearing impairment in the patients.
Conclusions: Our study unveils novel features on the phenotypic landscape of PRLTS and provides further evidence that the newly identified for PRLTS TWNK gene is involved in its pathogenesis.
Keywords: Auditory neuropathy; C10orf2; Hearing; Perrault syndrome; TWNK; Vestibulocochlear nerve; Whole exome sequencing.
Figures
Fig. 1
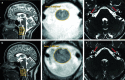
Brain and spine MRI in the proband. The left column shows sagittal T2 weighted images of the head and cervical spine (a proband, b control) with the measurements of spinal cord thickness at different levels. The middle column shows cross-section of the spinal cord (c proband, d control). The right column shows transverse heavy T2 weighted images of sub-millimeter slice thickness displaying the cochlear nerve, pointed with a red arrow (e proband, f control)
Fig. 2
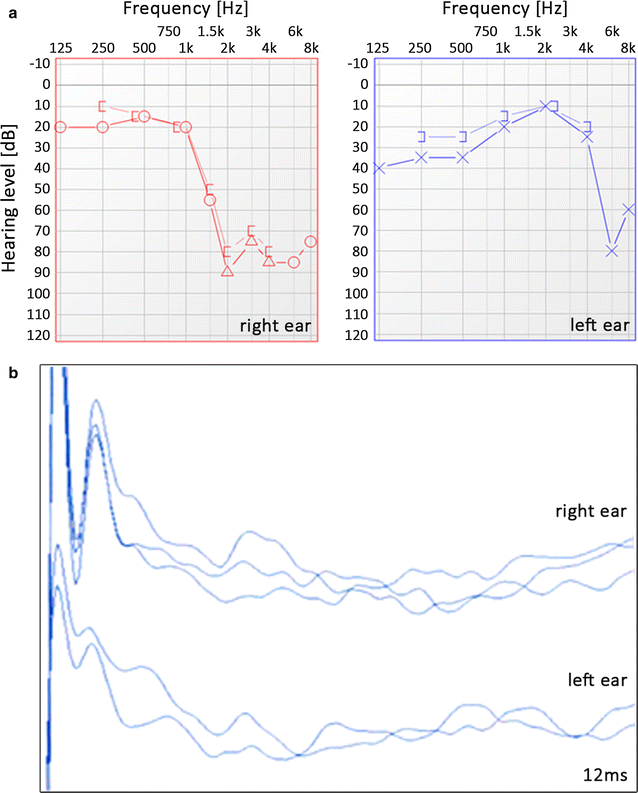
Pure tone audiometry (a) and ABRs (b) of the proband. a “O” and “X” symbols denote air conduction thresholds in the right and left ear, respectively and “Δ” denotes masked air conduction at high frequencies in the right ear; “[” or “]” denote masked bone conduction. b Shown are ABR recordings after click stimulus at an acoustic level of 90 dB normal hearing level (nHL) and presentation rate of 11/s
Fig. 3
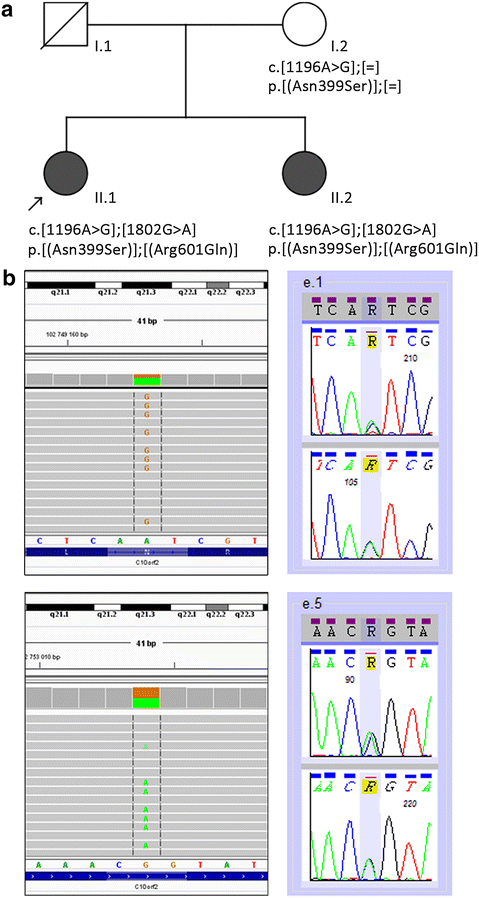
Identification of TWNK mutations in the analyzed families. a Pedigree of the investigated family. The proband is marked with an arrow. Black symbols indicate individuals affected with PRLTS5 and open symbols indicate unaffected individuals; diagonal line denotes the deceased father. The TWNK genotypes identified in the family members are reported at the cDNA and protein levels according to the HGVS-nomenclature (
; accessed 07/2016). b WES in the proband revealed an A>G transition (upper left panel) and G>A transition (lower left panel), corresponding to p.Asn399Ser (AAT>AGT) and p.Arg601Gln (CGG>CAG), respectively, in the TWNK gene. Direct Sanger sequencing of TWNK confirmed the presence of the two mutations (right panel). For each mutation sequencing of the forward (top) and the reverse (bottom) strand is shown
Fig. 4
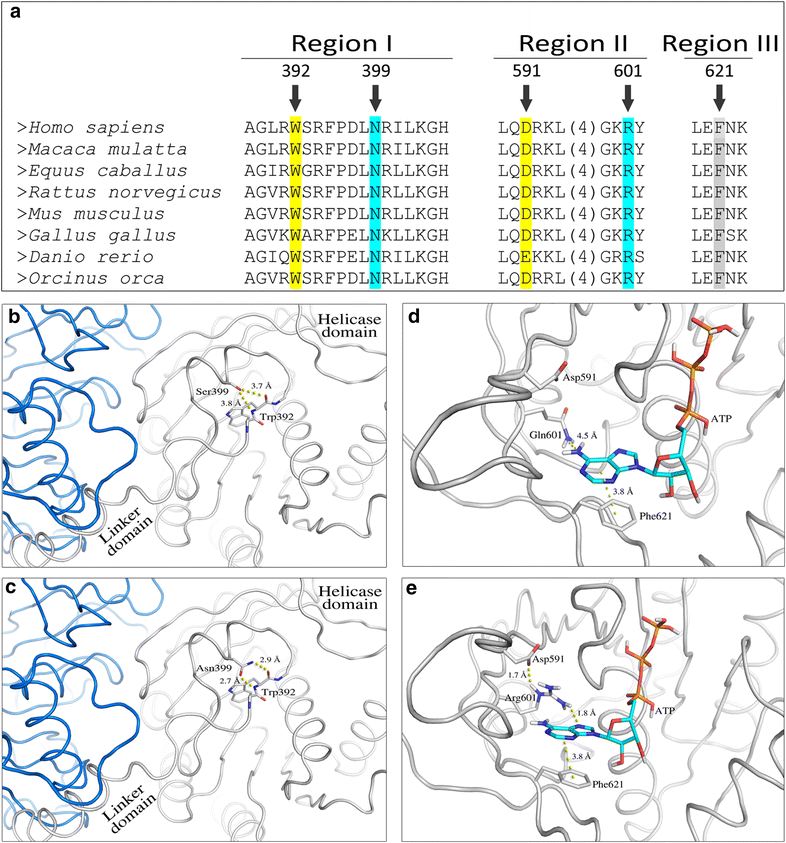
Multiple protein sequence alignment and 3D structure of the human Twinkle protein in regions encompassing p.Asn399Ser and p.Arg601Gln mutations. a Multiple protein sequence alignment of selected sequences. Two regions of the twinkle protein are shown: (i) the region joining the linker and the helicase domains (Region I) and (ii) the region involved in stabilizing the adenine ring of ATP (Regions II and III). The numbers above the alignment correspond to the amino acid position in the human protein sequence. (b–c) Two interacting monomers of the Twinkle protein are colored white and blue. Changes in the conformation of a region next to the linker domain in p.Asn399Ser mutant (b) and in wild type (c) proteins are shown. (d–e) Changes in hydrogen bonds network resulting in weakened ATP binding in the p.Arg601Gln mutant (d) as compared to wild type (e) protein are depicted
Similar articles
- Perrault syndrome with neurological features in a compound heterozygote for two TWNK mutations: overlap of TWNK-related recessive disorders.
Domínguez-Ruiz M, García-Martínez A, Corral-Juan M, Pérez-Álvarez ÁI, Plasencia AM, Villamar M, Moreno-Pelayo MA, Matilla-Dueñas A, Menéndez-González M, Del Castillo I. Domínguez-Ruiz M, et al. J Transl Med. 2019 Aug 28;17(1):290. doi: 10.1186/s12967-019-2041-x. J Transl Med. 2019. PMID: 31455392 Free PMC article. - Expanding the genotypic spectrum of Perrault syndrome.
Demain LA, Urquhart JE, O'Sullivan J, Williams SG, Bhaskar SS, Jenkinson EM, Lourenco CM, Heiberg A, Pearce SH, Shalev SA, Yue WW, Mackinnon S, Munro KJ, Newbury-Ecob R, Becker K, Kim MJ, O' Keefe RT, Newman WG. Demain LA, et al. Clin Genet. 2017 Feb;91(2):302-312. doi: 10.1111/cge.12776. Epub 2016 Apr 1. Clin Genet. 2017. PMID: 26970254 - Broadening the phenotype of the TWNK gene associated Perrault syndrome.
Fekete B, Pentelényi K, Rudas G, Gál A, Grosz Z, Illés A, Idris J, Csukly G, Domonkos A, Molnar MJ. Fekete B, et al. BMC Med Genet. 2019 Dec 18;20(1):198. doi: 10.1186/s12881-019-0934-4. BMC Med Genet. 2019. PMID: 31852434 Free PMC article. - An Application of NGS for Molecular Investigations in Perrault Syndrome: Study of 14 Families and Review of the Literature.
Lerat J, Jonard L, Loundon N, Christin-Maitre S, Lacombe D, Goizet C, Rouzier C, Van Maldergem L, Gherbi S, Garabedian EN, Bonnefont JP, Touraine P, Mosnier I, Munnich A, Denoyelle F, Marlin S. Lerat J, et al. Hum Mutat. 2016 Dec;37(12):1354-1362. doi: 10.1002/humu.23120. Epub 2016 Oct 7. Hum Mutat. 2016. PMID: 27650058 Review. - LARS2-Perrault syndrome: a new case report and literature review.
Carminho-Rodrigues MT, Klee P, Laurent S, Guipponi M, Abramowicz M, Cao-van H, Guinand N, Paoloni-Giacobino A. Carminho-Rodrigues MT, et al. BMC Med Genet. 2020 May 18;21(1):109. doi: 10.1186/s12881-020-01028-8. BMC Med Genet. 2020. PMID: 32423379 Free PMC article. Review.
Cited by
- CLPP Gene Variants Causing Perrault Syndrome Type 3 in Han Chinese Families: A Genotype-Phenotype Study.
Long X, Yang B, Wang W, Peng W, Wang X, Xiong W, Liu M, Yuan H, Lu Y. Long X, et al. Hum Genomics. 2025 May 23;19(1):60. doi: 10.1186/s40246-025-00762-5. Hum Genomics. 2025. PMID: 40410890 Free PMC article. - Middle-age-onset cerebellar ataxia caused by a homozygous TWNK variant: a case report.
Kume K, Morino H, Miyamoto R, Matsuda Y, Ohsawa R, Kanaya Y, Tada Y, Kurashige T, Kawakami H. Kume K, et al. BMC Med Genet. 2020 Mar 31;21(1):68. doi: 10.1186/s12881-020-01002-4. BMC Med Genet. 2020. PMID: 32234020 Free PMC article. - Perrault syndrome: a forgotten presentation for infertile women.
Alkhonezan M, Alkhonezan S, Al-Jaroudi D. Alkhonezan M, et al. Clin Case Rep. 2024 Nov 4;12(11):e9522. doi: 10.1002/ccr3.9522. eCollection 2024 Nov. Clin Case Rep. 2024. PMID: 39498437 Free PMC article. - TBC1D24 emerges as an important contributor to progressive postlingual dominant hearing loss.
Oziębło D, Leja ML, Lazniewski M, Sarosiak A, Tacikowska G, Kochanek K, Plewczynski D, Skarżyński H, Ołdak M. Oziębło D, et al. Sci Rep. 2021 May 13;11(1):10300. doi: 10.1038/s41598-021-89645-y. Sci Rep. 2021. PMID: 33986365 Free PMC article. - Genetic etiology of Perrault syndrome in Iranian families: first report from Iran and literature review.
Shokouhian E, Kahrizi K, Najmabadi H, Babanejad M. Shokouhian E, et al. J Appl Genet. 2025 Jan 23. doi: 10.1007/s13353-025-00940-0. Online ahead of print. J Appl Genet. 2025. PMID: 39847269
References
- Newman WG, Friedman TB, Conway GS. Perrault Syndrome. 1993. - PubMed
- Gispert S, Parganlija D, Klinkenberg M, Drose S, Wittig I, Mittelbronn M, Grzmil P, Koob S, Hamann A, Walter M, et al. Loss of mitochondrial peptidase Clpp leads to infertility, hearing loss plus growth retardation via accumulation of CLPX, mtDNA and inflammatory factors. Hum Mol Genet. 2013;22:4871–4887. doi: 10.1093/hmg/ddt338. - DOI - PMC - PubMed
- Pierce SB, Chisholm KM, Lynch ED, Lee MK, Walsh T, Opitz JM, Li W, Klevit RE, King MC. Mutations in mitochondrial histidyl tRNA synthetase HARS2 cause ovarian dysgenesis and sensorineural hearing loss of Perrault syndrome. Proc Natl Acad Sci USA. 2011;108:6543–6548. doi: 10.1073/pnas.1103471108. - DOI - PMC - PubMed
- Jenkinson EM, Rehman AU, Walsh T, Clayton-Smith J, Lee K, Morell RJ, Drummond MC, Khan SN, Naeem MA, Rauf B, et al. Perrault syndrome is caused by recessive mutations in CLPP, encoding a mitochondrial ATP-dependent chambered protease. Am J Hum Genet. 2013;92:605–613. doi: 10.1016/j.ajhg.2013.02.013. - DOI - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous