The molecular basis of endothelial cell plasticity - PubMed (original) (raw)
Review
The molecular basis of endothelial cell plasticity
Elisabetta Dejana et al. Nat Commun. 2017.
Abstract
The endothelium is capable of remarkable plasticity. In the embryo, primitive endothelial cells differentiate to acquire arterial, venous or lymphatic fates. Certain endothelial cells also undergo hematopoietic transition giving rise to multi-lineage hematopoietic stem and progenitors while others acquire mesenchymal properties necessary for heart development. In the adult, maintenance of differentiated endothelial state is an active process requiring constant signalling input. The failure to do so leads to the development of endothelial-to-mesenchymal transition that plays an important role in pathogenesis of a number of diseases. A better understanding of these phenotypic changes may lead to development of new therapeutic interventions.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
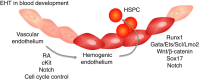
Figure 1. Schematic representation of endothelial-to-hematopoietic (EHT) transition and endothelial-to-mesenchymal (EndMT) transition during development.
During definitive hematopoiesis, a subset of endothelial cells is specified to become hemogenic (dark red), and these cells give rise to hematopoietic stem and progenitor cells (HSPC) via EHT. The specification of hemogenic endothelial cells requires retinoic acid (RA) signalling, which leads to upregulation of c-Kit. Notch is activated downstream of c-Kit expression and controls endothelial cell cycle to enable hemogenic specification via mechanisms that are still unclear. The subsequent generation of HSPC requires transcription factor Runx1, and binding partners Gata, Ets, Scl and Lmo-2. Other factors involved in this process include Wnt/β-catenin, Sox17 and Notch, whose molecular roles and interactions are under study.
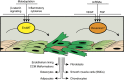
Figure 2. Schematic representation of endothelial-to-mesenchymal transition in the adult.
In the adult, endothelial cells (flesh coloured) deprived of growth factors or exposed to inflammatory cytokines may undergo EndMT (see light and intense green cells representing the progression to EndMT) and acquire characteristics of fibroblasts, smooth muscle cells, osteocytes, adipocytes, chondrocytes or form vascular malformations such as CCM. Re-establishment of endothelial homeostasis by exposure to growth factors or to specific miRNAs may revert the mesenchymal phenotype (from intense green to flesh coloured cells).
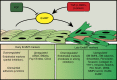
Figure 3. EndMT markers.
Endothelial cells may undergo EndMT either from growth factor deprivation (FGF) or from activation of β-catenin, TGFβ, BMP pathways. EndMT progresses through different steps. The early endothelial response is characterized by a partial downregulation of endothelial markers, junction dismantling and up-regulation of some early mesenchymal markers. At later times, expression of endothelial markers further declines while more mesenchymal markers including matrix proteins, metallo-proteases or cytoskeletal proteins are up-regulated.
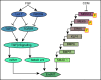
Figure 4. Signalling pathways inducing EndMT.
Endothelial cells undergo EndMT as a result of the loss of FGF or CCM protective inputs. In the former case (left signalling sequence) reduction in let-7 levels results in increase expression of TGFβ family members' expression and activation of TGFβ signalling. MiR20a is induced by FGF and contributes to inhibition of EndMT by inhibiting TGFβ receptors signalling. Conversely, miR 21 is downstream TGFβ signalling and reduces EndMT development. In the latter (right signalling sequence), the loss of CCM inhibitory input activate MEKK3/MEK5/ERK5 cascade resulting in induction of BMP/Smad signalling.
References
- Aird W. C. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ. Res. 100, 158–173 (2007) One of the first papers underlining endothelial heterogeneity. - PubMed
- Chung A. S. & Ferrara N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 27, 563–584 (2011). - PubMed
- De Val S. & Black B. L. Transcriptional control of endothelial cell development. Dev. Cell 16, 180–195 (2009) Although a few key regulators of endothelial cell differentiation have been identified, including the Ets family members, the molecular regulation of this process is still largely undefined. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- R01 HL096360/HL/NHLBI NIH HHS/United States
- P01 HL107205/HL/NHLBI NIH HHS/United States
- R01 HL053793/HL/NHLBI NIH HHS/United States
- UH2 EB017103/EB/NIBIB NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- R01 EB016629/EB/NIBIB NIH HHS/United States
- R01 HL128064/HL/NHLBI NIH HHS/United States
- UG3 EB025765/EB/NIBIB NIH HHS/United States
- UH3 EB017103/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources