Real-time imaging of sodium glucose transporter (SGLT1) trafficking and activity in single cells - PubMed (original) (raw)
Real-time imaging of sodium glucose transporter (SGLT1) trafficking and activity in single cells
Chiara Ghezzi et al. Physiol Rep. 2017 Feb.
Abstract
The processes controlling targeting of glucose transporters to apical and basolateral membranes of polarized cells are complex and not-well understood. We have engineered SGLT1 and GLUT4 constructs linked to fluorescent proteins to highlight the differences in transporter expression and trafficking, in real time, in different cell types. Activity was assessed in parallel using a FRET glucose sensor. In COS cells and HEK cells, SGLT1 was distributed between the plasma membrane and intracellular compartments, but there was little expression in CHO cells. Trafficking was investigated using the lysosome inhibitors NH4Cl (10 mmol/L) and chloroquine (150 _μ_mol/L) and the proteasome inhibitors MG-262 (1 _μ_mol/L) and lactacystin (5 _μ_mol/L). Lysosome inhibitors caused SGLT1 accumulation into intracellular bodies, whereas proteasome inhibitors induced SGLT1 accumulation in the plasma membrane, even in CHO cells. Our data suggest that a fraction of SGLT1 is rapidly degraded by lysosomes and never reached the plasma membrane; another fraction reaches the membrane and is subsequently degraded by lysosomes following internalization. The latter process is regulated by the ubiquitin/proteasome pathway, acting at a late stage of the lysosomal pathway. Using the cholesterol inhibitor M_β_CD (3 mmol/L), a dominant negative dynamin (K44A) and caveolin, we showed that SGLT1 internalization is lipid raft-mediated, but caveolin-independent. In contrast, GLUT4 internalization is dynamin-dependent, but cholesterol-independent. The physiological relevance of these data is discussed in terms of differential membrane compartmentalization of the transporters and expression under stress conditions.
Keywords: Endocytosis; SGLT1; lysosome; proteasome.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures
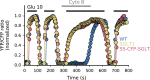
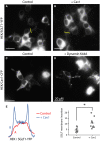
Figure 1
SGLT
1‐dependent glucose transport in
COS
cells. The differences in
FRET
ratios in Fig 1 illustrate how overexpression of wt
SGLT
1 and fluorescent construct
SS
‐
CFP
‐
SGLT
1 modulate glucose entry in
COS
cells expressing the
FRET
‐ based glucose sensor Flip 600 _μ_mol/L. In this panel, three traces are superimposed that were obtained in three sets of experiments with
COS
cells expressing either wt
SGLT
1,
SS
‐
CFP
‐
SGLT
1 or no
SGLT
1. At the beginning of the traces, glucose entry resulting from the addition of 10 mmol/L glucose to the bath was mediated by both endogenous
GLUT
s and overexpressed
SGLT
1. In all three cases, glucose efflux following bath glucose removal was mediated via endogenous
GLUT
s. In the middle of the traces, 10 _μ_mol/L CytoB was added to the bath to block endogenous
GLUT
s. Under these conditions, addition of 10 mmol/L glucose to the bath evoked glucose entries of similar amplitude with cells expressing wt
SGLT
1 and
SS
‐
CFP
‐
SGLT
1, indicating that
SS
‐
CFP
‐
SGLT
1 is fully functional. The fact that there is no glucose entry in cells that do not overexpress
SGLT
1, suggest that there is no detectable endogenous
SGLT
activity in
COS
cells.
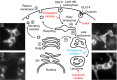
Figure 2
SGLT
1 trafficking uses a classical pathway in
HEK
and
COS
cells. Depending on the experimental conditions, our data show that
SGLT
1‐
YFP
may be found in the trans Golgi network (
TGN
) (1), vesicles (2), the plasma membrane (3), or endosome (4). These images suggest that insertion and retrieval of
SGLT
1 in and out of the membrane follows a classical pathway. This diagram also specifies the modulator of endocytosis and degradation that were used and their potential sites of action. The letter “A” identifies the trafficking of proteins from the
TGN
to lysosome and “B” identifies the trafficking of endocytosed proteins from the plasma membrane to lysosome. The mode of action of the
GTP
ase dynamin is also identified in this diagram.
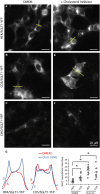
Figure 3
The cholesterol inhibitor M_β_
CD
enhances insertion of
SGLT
1 in the plasma membrane. In
HEK
cells (A) and
COS
cells (B), and under normal culture conditions (
DMEM
),
SGLT
1‐
YFP
is primarily located inside the cells in small vesicles and in larger structures identified as endosomes. In contrast, there is almost no
SGLT
1 expression in
CHO
cells. An image of a transfected
CHO
cells is shown in (C) for reference, but only 10–12 transfected cells were found in an entire 1.5 cm dish per experiment. Incubation overnight with the cholesterol inhibitor M_β_
CD
(3 mmol/L) facilitates insertion of
SGLT
1 in the membrane in
HEK
cells (D) and
COS
cells (E). In
CHO
cells, incubation with M_β_
CD
had no effect (F) and the number of transfected cells remained very low. In (G), the left panels show fluorescence intensity profiles for each condition. The graph in the right panel shows a quantification of
SGLT
1‐
YFP
insertion in the plasma membrane, using five different intensity profiles for each condition (see Experimental Procedures for details).
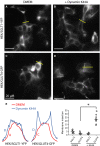
Figure 4
In
HEK
cells, the dynamin dominant negative K44A facilitates
GLUT
4 insertion in the plasma membrane, but has no effect on
SGLT
1 trafficking. Panels A (
SGLT
1‐
YFP
expressed in the absence of K44A) and B (co‐expression of
SGLT
1 with K44A) shows that
SGLT
1 remains associated with intracellular compartments when co‐expressed with K44A (B). In contrast,
GLUT
4‐
YFP
, which is also mostly localized to intracellular compartments under normal conditions (C) is directed to the plasma membrane when co‐expressed with K44A (D). Panel E shows the fluorescence intensity profiles for each condition. The graph in the right panel shows quantifications of
SGLT
1‐
YFP
and
GLUT
4‐
GFP
insertion in the plasma membrane.
Figure 5
Caveolin 1 (Cav1) does not regulate
SGLT
1 trafficking in
HEK
and
COS
cells. Lipid raft‐dependent endocytosis may be caveolin‐dependent or independent. Overexpression of Cav1 with
SGLT
1‐
YFP
in
HEK
cells causes accumulation of
SGLT
1 in the plasma membrane (B and E). However, as shown in (F), there is no co‐localization between
SGLT
1‐
YFP
and Cav1‐
CFP
. When expressed alone, Cav1‐
CFP
is targeted to “hot spots” in the plasma membrane, but is also localized to intracellular compartments (C). When co‐expressed with the dynamin‐dominant negative K44A, there is a strong accumulation of Cav1‐
CFP
in the plasma membrane (D). These data contrast with that in Panels A and B of Fig 4 where K44A had no effect on
SGLT
1 trafficking. Panel E shows the fluorescence intensity profiles for (A) and (B). The graph in the right panel shows quantifications of
SGLT
1‐
YFP
insertion in the plasma membrane for (A) and (B).
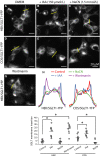
Figure 6
Effects of metabolic inhibitors on
SGLT
1 trafficking. Vesicle exocytosis and endocytosis are processes that require energy. We tested the effects of inhibitors of
ATP
production by glycolysis (
IAA
) and
TCA
cycle (Na
CN
) on
SGLT
1 trafficking. Incubation with 150 _μ_mol/L
IAA
caused
SGLT
1 insertion in the plasma membrane within 1–2 h in
HEK
cells (B) and
COS
cells (E). In contrast, incubation with Na
CN
(1.5 mmol/L) had no effect on
SGLT
1 trafficking in either
HEK
(C) and
COS
cells (F). The inhibitor of
PI
3 kinase, wortmannin causes accumulation of
SGLT
1 in the plasma membrane (G), suggesting that the effect of
ATP
is due in part to activation of
PI
3 kinase. Panel H shows the fluorescence intensity profiles for each of the condition illustrated in Panels (A) to (G). The lower graph shows quantifications of
SGLT
1‐
YFP
insertion in the plasma membrane for each condition from (A) to (G).
Figure 7
Proteosomal‐ and lysosomal‐dependent degradation of
SGLT
‐
YFP
in
CHO
,
HEK
, and
COS
cells. For the series of experiments depicted in this figure, we have used the lysosome inhibitors chloroquine (150 _μ_mol/L) and
NH
4Cl (10 mmol/L) and the proteasome inhibitors
MG
262 (1 _μ_mol/L) and lactacystine (5 _μ_mol/L). Panel A illustrates, as previously shown in Fig 3C, that there is almost no expression of
SGLT
1 in
CHO
cells under normal culture conditions (A). Addition of the lysosomal inhibitor chloroquine for 4–8 h causes
SGLT
1‐
YFP
accumulation in what appears to be the membrane of large vesicles identified as lysosomes (B). Incubation with
NH
4Cl for the same period of time had a similar, but perhaps lesser effect (not shown). Incubation with the proteasome inhibitor
MG
262 also causes expression of
SGLT
1‐
YFP
in
CHO
cells, but in this case, a large fraction of the protein was targeted to the plasma membrane (C). Bar graph data in panel (J) indicate that
SGLT
1 increased insertion of
SGLT
1 in the plasma membrane in response to
MG
262 was not associated with increased glucose uptake. The same experiments carried out in
HEK
and
COS
cells showed similar results. Incubation with the lysosome inhibitor, chloroquine, directs
SGLT
1‐
YFP
to large lysosomal vesicles within 4 h in
HEK
cells (E) and
COS
cells (H). Incubation with the proteasome inhibitor
MG
262 enhanced
SGLT
1‐
YFP
insertion in the plasma membrane after 4–6 h in
HEK
cells (F) and
COS
cells (I). However, in the cases of
HEK
and
COS
cells, most of
SGLT
1‐
YFP
was targeted to the plasma membrane (and the Golgi) and there was little fluorescence associated with intracellular compartments. Incubation with the proteasome inhibitor lactacystin had the same effects.
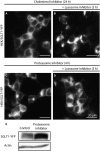
Figure 8
Does
SGLT
1 traffic via the plasma membrane prior to degradation by lysosomes? To test whether
SGLT
1 traffics through the plasma membrane prior to be degraded by lysosomes, we first incubated overnight
HEK
cells expressing
SGLT
1‐
YFP
with the cholesterol inhibitor (M_β_
CD
) (A) and added after that the lysosome inhibitor chloroquine to the incubation medium (B). After incubation with M_β_
CD
, a large fraction of
SGLT
1‐
YFP
is targeted to the plasma membrane (A), but after addition of the lysosome inhibitor, the newly synthesized
SGLT
1 is directed to lysosome, with no additional insertion in the plasma membrane (B). These data suggest that a large fraction of the newly synthesized
SGLT
1 is targeted to lysosomes. We did the same experiments using the proteasome inhibitor. In this case,
HEK
cells expressing
SGLT
1 were first incubated with
MG
262 for 4–6 h to target the transporter to the plasma membrane (C), then the lysosome inhibitor choloroquine was added (D). Data in (D) show that, following addition of the inhibitor,
SGLT
1‐
YFP
was not targeted to lysosomes, but instead
SGLT
1‐
YFP
insertion in the plasma membrane was sustained, while labeling of the Golgi increased. Western blot in (E) shows an increase of 34.5% (n = 2) of
SGLT
1‐
YFP
protein level after 8 h incubation in the presence of the proteasome inhibitor
MG
262 (1 _μ_mol/L). Actin (lower band) was used to normalize the protein levels obtained with and without incubation with
MG
Figure 9
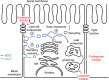
Model for
SGLT
1 and
GLUT
4 trafficking in polarized epithelial cells. Based on our data as well as those of others and the model of Rodriguez‐Boulan (Rodriguez‐Boulan et al. 2005; Lakkaraju and Rodriguez‐Boulan 2007), we propose that
SGLT
1 traffics from the Golgi (1) to the basolateral membrane (3) before being internalized to endosome (4) and finally inserted into the apical membrane. This trafficking pathway has been denoted the transcytotic pathway, in opposition to the direct pathway that takes the proteins from the Golgi to the apical membrane. In this model, we assume that the basolateral to apical membrane trafficking is lipid‐raft mediated. It follows based on this simple assumption that
SGLT
1, which internalization is cholesterol‐dependent, would translocate to the apical membrane, while
GLUT
4, which internalization is not cholesterol‐mediated, would remain in the basolateral membrane.
Similar articles
- Novel natural and synthetic inhibitors of solute carriers SGLT1 and SGLT2.
Oranje P, Gouka R, Burggraaff L, Vermeer M, Chalet C, Duchateau G, van der Pijl P, Geldof M, de Roo N, Clauwaert F, Vanpaeschen T, Nicolaï J, de Bruyn T, Annaert P, IJzerman AP, van Westen GJP. Oranje P, et al. Pharmacol Res Perspect. 2019 Jul 30;7(4):e00504. doi: 10.1002/prp2.504. eCollection 2019 Aug. Pharmacol Res Perspect. 2019. PMID: 31384471 Free PMC article. - Cardiac ischemia-reperfusion injury under insulin-resistant conditions: SGLT1 but not SGLT2 plays a compensatory protective role in diet-induced obesity.
Yoshii A, Nagoshi T, Kashiwagi Y, Kimura H, Tanaka Y, Oi Y, Ito K, Yoshino T, Tanaka TD, Yoshimura M. Yoshii A, et al. Cardiovasc Diabetol. 2019 Jul 1;18(1):85. doi: 10.1186/s12933-019-0889-y. Cardiovasc Diabetol. 2019. PMID: 31262297 Free PMC article. - Development of a novel non-radioactive cell-based method for the screening of SGLT1 and SGLT2 inhibitors using 1-NBDG.
Chang HC, Yang SF, Huang CC, Lin TS, Liang PH, Lin CJ, Hsu LC. Chang HC, et al. Mol Biosyst. 2013 Aug;9(8):2010-20. doi: 10.1039/c3mb70060g. Epub 2013 May 8. Mol Biosyst. 2013. PMID: 23657801 - [Regulatory mechanisms of intracellular distribution of Na+-dependent glucose transporter and the role in recovery from cellular injury].
Ikari A. Ikari A. Yakugaku Zasshi. 2004 Dec;124(12):959-64. doi: 10.1248/yakushi.124.959. Yakugaku Zasshi. 2004. PMID: 15577265 Review. Japanese. - The Na+-D-glucose cotransporters SGLT1 and SGLT2 are targets for the treatment of diabetes and cancer.
Koepsell H. Koepsell H. Pharmacol Ther. 2017 Feb;170:148-165. doi: 10.1016/j.pharmthera.2016.10.017. Epub 2016 Oct 20. Pharmacol Ther. 2017. PMID: 27773781 Review.
Cited by
- Canagliflozin, an Inhibitor of the Na+-Coupled D-Glucose Cotransporter, SGLT2, Inhibits Astrocyte Swelling and Brain Swelling in Cerebral Ischemia.
Shim B, Stokum JA, Moyer M, Tsymbalyuk N, Tsymbalyuk O, Keledjian K, Ivanova S, Tosun C, Gerzanich V, Simard JM. Shim B, et al. Cells. 2023 Sep 6;12(18):2221. doi: 10.3390/cells12182221. Cells. 2023. PMID: 37759444 Free PMC article. - Role of Caveolin-1 in Diabetes and Its Complications.
Haddad D, Al Madhoun A, Nizam R, Al-Mulla F. Haddad D, et al. Oxid Med Cell Longev. 2020 Jan 27;2020:9761539. doi: 10.1155/2020/9761539. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32082483 Free PMC article. Review. - Drosophila Solute Carrier 5A5 Regulates Systemic Glucose Homeostasis by Mediating Glucose Absorption in the Midgut.
Li Y, Wang W, Lim HY. Li Y, et al. Int J Mol Sci. 2021 Nov 17;22(22):12424. doi: 10.3390/ijms222212424. Int J Mol Sci. 2021. PMID: 34830305 Free PMC article. - A high-throughput screen identifies that CDK7 activates glucose consumption in lung cancer cells.
Ghezzi C, Wong A, Chen BY, Ribalet B, Damoiseaux R, Clark PM. Ghezzi C, et al. Nat Commun. 2019 Nov 29;10(1):5444. doi: 10.1038/s41467-019-13334-8. Nat Commun. 2019. PMID: 31784510 Free PMC article. - Structure and mechanism of the SGLT family of glucose transporters.
Han L, Qu Q, Aydin D, Panova O, Robertson MJ, Xu Y, Dror RO, Skiniotis G, Feng L. Han L, et al. Nature. 2022 Jan;601(7892):274-279. doi: 10.1038/s41586-021-04211-w. Epub 2021 Dec 8. Nature. 2022. PMID: 34880492 Free PMC article.
References
- Al‐Hasani, H. , Hinck C. S., and Cushman S. W.. 1998. Endocytosis of the glucose transporter GLUT4 is mediated by the GTPase dynamin. J. Biol. Chem. 273:17504–17510. - PubMed
- Alwan, H. A. , van Zoelen E. J., and van Leeuwen J. E.. 2003. Ligand‐induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome‐dependent EGFR deubiquitination. J. Biol. Chem. 278:35781–35790. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous