Effects of Moringa oleifera aqueous leaf extract in alloxan induced diabetic mice - PubMed (original) (raw)
Review
Effects of Moringa oleifera aqueous leaf extract in alloxan induced diabetic mice
Muobarak J Tuorkey. Interv Med Appl Sci. 2016 Sep.
Abstract
Objective: There is a lack of knowledge regarding the underlying mechanisms of the antidiabetic activity of Moringa oleifera. This study investigates the antidiabetic effect of M. oleifera and its impact on the immune tolerance.
Methods: Alloxan-induced diabetes model for mice was used. A dose of 100 mg/kg of Moringa extract was orally administered to diabetic treated mice. Glucose and insulin levels were evaluated to calculate insulin resistance. Total antioxidant capacity (TAC), creatinine, and blood urea nitrogen (BUN) levels were measured. The relative percentage of CD44, CD69, and IFN-γ was investigated by flow cytometry.
Results: In diabetic mice, insulin resistance by homeostasis model assessment of insulin resistance (HOMA-IR) was increased 4.5-fold than in the control group, and HOMA-IR was decreased 1.3-fold in the Moringa treatment group. The level of TAC was declined 1.94-fold in diabetic mice, and increased 1.67-fold in diabetic treated group. In diabetic mice, creatinine and BUN levels were significantly reduced 1.42- and 1.2-fold, respectively, in Moringa treatment mice. The relative percentage of CD44 was not changed in diabetic mice, but the relative percentage of CD69 was found to be increased. INF-γ was decreased 2.4-fold in diabetic mice and elevated in treated groups.
Conclusion: Moringa may ameliorate insulin resistance, increase TAC, and improve immune tolerance.
Keywords: blood urea nitrogen; creatinine; immune tolerance; insulin resistance; total antioxidant capacity.
Figures
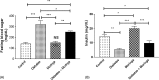
Fig. 1.
Fasting glucose (A) and insulin (B) levels in different mice involved in this study. Data were expressed as mean ± SE of 10 mice in each group. *P < 0.05, **P < 0.01, and ***P < 0.001, NS: statistically non-significant
Fig. 2.
HOMA-IR analysis in different mice involved in this study. HOMA-IR was calculated from glucose (mg/dL) and insulin (μU/mL) levels using the following formula: HOMA = fasting glucose value (mg/dL) × fasting insulin value (μU/mL)/405. Data were expressed as mean ± SE of 10 mice in each group. *P < 0.05, **P < 0.01, and ***P < 0.001, NS: statistically non-significant
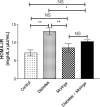
Fig. 3.
Total antioxidant capacity in different mice involved in this study. Data were expressed as mean ± SE of 10 mice in each group. *P < 0.05, **P < 0.01, and ***P < 0.001, NS: statistically non-significant
Fig. 4.
The level of creatinine (A) and blood urea nitrogen (B) levels in different mice involved in this study. Data were expressed as mean ± SE of 10 mice in each group. *P < 0.05, **P < 0.01, and ***P < 0.001, NS: statistically non-significant
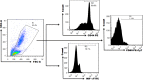
Fig. 5.
The Fluorescence Minus One gating boundaries for CD44, IFN-γ, and CD69 molecules
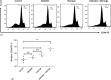
Fig. 6.
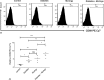
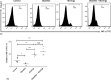
Representative histograms showing the expression of the CD44 molecules in the peripheral blood of different mice involved in this study (A). A representative histogram showing the relative percentage of CD44 molecules (B). Data were expressed as mean ± SE of five mice in each group. *P < 0.05, **P < 0.01, and ***P < 0.001, NS: statistically non-significant
Fig. 7.
Representative histograms showing the expression of the CD69 molecules in the peripheral blood of different mice involved in this study (A). A representative histogram showing the relative percentage of CD69 molecules (B). Data were expressed as mean ± SE of five mice in each group. *P < 0.05, **P < 0.01, and ***P < 0.001, NS: statistically non-significant
Fig. 8.
Representative histograms showing the expression of IFN-γ molecules in the peripheral blood of different mice involved in this study (A). A representative histogram showing the relative percentage CD69 molecules (B). Data were expressed as mean ± SE of five mice in each group. *P < 0.05, **P < 0.01, and ***P < 0.001, NS: statistically non-significant
Similar articles
- Chronic administration of ethanol leaf extract of Moringa oleifera Lam. (Moringaceae) may compromise glycaemic efficacy of Sitagliptin with no significant effect in retinopathy in a diabetic rat model.
Olurishe C, Kwanashie H, Zezi A, Danjuma N, Mohammed B. Olurishe C, et al. J Ethnopharmacol. 2016 Dec 24;194:895-903. doi: 10.1016/j.jep.2016.10.065. Epub 2016 Oct 24. J Ethnopharmacol. 2016. PMID: 27789327 - Moringa oleifera leaf extract ameliorates alloxan-induced diabetes in rats by regeneration of β cells and reduction of pyruvate carboxylase expression.
Abd El Latif A, El Bialy Bel S, Mahboub HD, Abd Eldaim MA. Abd El Latif A, et al. Biochem Cell Biol. 2014 Oct;92(5):413-9. doi: 10.1139/bcb-2014-0081. Epub 2014 Sep 4. Biochem Cell Biol. 2014. PMID: 25289966 - Hypoglycemic Assessment of Aqueous Leaf Extract of Moringa oleifera on Diabetic Wistar Rats.
Amina EE, Adisa JO, Gamde SM, Omoruyi EB, Kwaambwa HM, Mwapagha LM. Amina EE, et al. Biochem Res Int. 2024 Oct 23;2024:9779021. doi: 10.1155/2024/9779021. eCollection 2024. Biochem Res Int. 2024. PMID: 39478982 Free PMC article. - Insulin-like plant proteins as potential innovative drugs to treat diabetes-The Moringa oleifera case study.
Paula PC, Oliveira JTA, Sousa DOB, Alves BGT, Carvalho AFU, Franco OL, Vasconcelos IM. Paula PC, et al. N Biotechnol. 2017 Oct 25;39(Pt A):99-109. doi: 10.1016/j.nbt.2016.10.005. Epub 2016 Oct 11. N Biotechnol. 2017. PMID: 27737801 Review. - Flavonoid from Moringa oleifera leaves revisited: A review article on in vitro, in vivo, and in silico studies of antidiabetic insulin-resistant activity.
Setyani W, Murwanti R, Sulaiman TNS, Hertiani T. Setyani W, et al. J Adv Pharm Technol Res. 2023 Oct-Dec;14(4):283-288. doi: 10.4103/JAPTR.JAPTR_290_23. Epub 2023 Oct 30. J Adv Pharm Technol Res. 2023. PMID: 38107449 Free PMC article. Review.
Cited by
- Moringa oleifera Lam.: a comprehensive review on active components, health benefits and application.
Su X, Lu G, Ye L, Shi R, Zhu M, Yu X, Li Z, Jia X, Feng L. Su X, et al. RSC Adv. 2023 Aug 15;13(35):24353-24384. doi: 10.1039/d3ra03584k. eCollection 2023 Aug 11. RSC Adv. 2023. PMID: 37588981 Free PMC article. Review. - Effects of Drying Temperature and Solvents on In Vitro Diabetic Wound Healing Potential of Moringa oleifera Leaf Extracts.
Muzammil S, Neves Cruz J, Mumtaz R, Rasul I, Hayat S, Khan MA, Khan AM, Ijaz MU, Lima RR, Zubair M. Muzammil S, et al. Molecules. 2023 Jan 11;28(2):710. doi: 10.3390/molecules28020710. Molecules. 2023. PMID: 36677768 Free PMC article. - Moringa Genus: A Review of Phytochemistry and Pharmacology.
Abd Rani NZ, Husain K, Kumolosasi E. Abd Rani NZ, et al. Front Pharmacol. 2018 Feb 16;9:108. doi: 10.3389/fphar.2018.00108. eCollection 2018. Front Pharmacol. 2018. PMID: 29503616 Free PMC article. Review. - Integrated Network Pharmacology Analysis and Experimental Validation to Reveal the Mechanism of Anti-Insulin Resistance Effects of Moringa oleifera Seeds.
Huang Q, Liu R, Liu J, Huang Q, Liu S, Jiang Y. Huang Q, et al. Drug Des Devel Ther. 2020 Oct 2;14:4069-4084. doi: 10.2147/DDDT.S265198. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33116398 Free PMC article. - Polyphenol Extract of Moringa Oleifera Leaves Alleviates Colonic Inflammation in Dextran Sulfate Sodium-Treated Mice.
Zhang Y, Peng L, Li W, Dai T, Nie L, Xie J, Ai Y, Li L, Tian Y, Sheng J. Zhang Y, et al. Evid Based Complement Alternat Med. 2020 Nov 24;2020:6295402. doi: 10.1155/2020/6295402. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33299453 Free PMC article.
References
- Brownlee M: Biochemistry and molecular cell biology of diabetic complications. Nature 414, 813–820 (2001) - PubMed
- Stevens MJ: Redox-based mechanisms in diabetes. Antioxid Redox Signal 7, 1483–1485 (2005) - PubMed
- Krentz AJ, Fujioka K, Hompesch M: Evolution of pharmacological obesity treatments: Focus on adverse side-effect profiles. Diabetes Obes Metab 18, 558–570 (2016) - PubMed
- Jongrungruangchok S, Bunrathep S, Songsak T: Nutrients and minerals content of eleven different samples of Moringa oleifera cultivated in Thailand. J Health Res 24, 123–127 (2010)
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous