Triethylene glycol, an active component of Ashwagandha (Withania somnifera) leaves, is responsible for sleep induction - PubMed (original) (raw)
Triethylene glycol, an active component of Ashwagandha (Withania somnifera) leaves, is responsible for sleep induction
Mahesh K Kaushik et al. PLoS One. 2017.
Abstract
Insomnia is the most common sleep complaint which occurs due to difficulty in falling asleep or maintaining it. Most of currently available drugs for insomnia develop dependency and/or adverse effects. Hence natural therapies could be an alternative choice of treatment for insomnia. The root or whole plant extract of Ashwagandha (Withania somnifera) has been used to induce sleep in Indian system of traditional home medicine, Ayurveda. However, its active somnogenic components remain unidentified. We investigated the effect of various components of Ashwagandha leaf on sleep regulation by oral administration in mice. We found that the alcoholic extract that contained high amount of active withanolides was ineffective to induce sleep in mice. However, the water extract which contain triethylene glycol as a major component induced significant amount of non-rapid eye movement sleep with slight change in rapid eye movement sleep. Commercially available triethylene glycol also increased non-rapid eye movement sleep in mice in a dose-dependent (10-30 mg/mouse) manner. These results clearly demonstrated that triethylene glycol is an active sleep-inducing component of Ashwagandha leaves and could potentially be useful for insomnia therapy.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
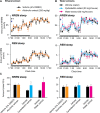
Fig 1. Oral administration of various extracts from Ashwagandha leaves in mice and their effect on sleep-wakefulness.
(A) Hourly plots of NREM and REM sleep after p.o. administration of vehicle (black circles) or alcoholic extract of Ashwagandha leaves (orange circles; n = 5). (B) Hourly plots of NREM and REM sleep after p.o. administration of vehicle (black circles) or water extracts of Ashwagandha leaves; cyclodextrin extract (blue circle) and water extract (magenta circles; n = 6). Arrows indicate time of p.o. administration. Black and white horizontal bars indicate 12 h dark and 12 h light period. (C) Changes in total amount of NREM (left graph) and REM sleep (right graph) during dark phase after p.o. administration of vehicle (black bars) and various Ashwagandha leaf extracts (colored bars). All administrations were done at the onset of dark period (17:00 h). Data presented as mean ± SEM; *p≤0.05, **p≤0.01 vs vehicle by using paired t-test.
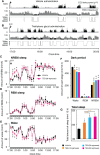
Fig 2. Dose-dependent increase in NREM sleep and decrease in wakefulness by p.o. administration of TEG in mice.
(A, B) Typical examples of EEG delta power (0.5–4 Hz), EMG integral, and hypnograms of a mouse after p.o. administration of vehicle (A) or TEG (B). Hypnograms represent concatenated 10-sec epochs of EEG/EMG activity, scored as wake, REM, and NREM sleep. Three hours after p.o. administration are shown. Wake, REM are shown in gray while NREM sleep shown in black. (C, D and E) Hourly plots of NREM, (C), REM sleep (D) and wake (E) in mice after p.o. administration of vehicle (black circles) and TEG (30 mg/mouse; magenta circles). Significant increase in NREM sleep and decrease in wake are apparent in the graphs at least up to 4 h after TEG administration. Black and white horizontal bars indicate 12 h dark and 12 h light period. (F) Total amount of wake, REM, and NREM sleep over 12 h dark period after vehicle (black bars) and various doses of TEG (colored bars) administration. There was a dose-dependent increase in NREM sleep with concomitant decrease in wake. (G) Graph shows total amount of sleep during dark phase (12 h) following vehicle (black bar) and various doses of TEG (color bars) administration. All administrations were done at the onset of dark period (17:00 h). Data presented as mean ± SEM; n = 6; *p≤0.05, **p≤0.01 vs vehicle by using paired t-test for time course data (C, D and E), and one-way ANOVA followed by least square difference (LSD) post-hoc test for dose-response data (F and G). # #p≤0.01 vs TEG (10 mg/head).
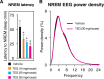
Fig 3. TEG decreased NREM sleep onset latency and induces physiological sleep.
(A), Graph shows changes in NREM sleep onset latency after vehicle (black bar) and various doses of TEG (color bars) administration. (B), Graph shows NREM EEG power density over 12 h dark phase following vehicle (black line) and TEG (magenta line) administration in mice. Data presented as mean ± SEM; n = 6; *p≤0.05, **p≤0.01 vs vehicle by one-way ANOVA followed by least square difference (LSD) post-hoc test.
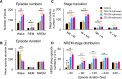
Fig 4. TEG induces sleep by targeting sleep generation mechanism.
Graphs represent changes in number of episodes (A), stage duration (B) of wake, REM and NREM sleep during 12 h dark phase after p.o. administration of vehicle (black bars) and various doses of TEG (colored bars). (C) Stage transition between wake, REM, and NREM sleep in mice after vehicle or TEG treatment. (D) NREM sleep stage distribution was calculated by averaging various NREM episodes within specific time durations, in mice after vehicle and TEG administration. Data presented as mean ± SEM; n = 6; *p≤0.05, **p≤0.01 vs vehicle; **+**p≤0.05, **++**p≤0.01 vs 10 mg/mouse TEG, by using one-way ANOVA followed by least square difference (LSD) post-hoc test. NR: NREM sleep; W: Wake; R: REM sleep.
Similar articles
- Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-blind, Randomized, Placebo-controlled Study.
Langade D, Kanchi S, Salve J, Debnath K, Ambegaokar D. Langade D, et al. Cureus. 2019 Sep 28;11(9):e5797. doi: 10.7759/cureus.5797. Cureus. 2019. PMID: 31728244 Free PMC article. - Clinical evaluation of the pharmacological impact of ashwagandha root extract on sleep in healthy volunteers and insomnia patients: A double-blind, randomized, parallel-group, placebo-controlled study.
Langade D, Thakare V, Kanchi S, Kelgane S. Langade D, et al. J Ethnopharmacol. 2021 Jan 10;264:113276. doi: 10.1016/j.jep.2020.113276. Epub 2020 Aug 17. J Ethnopharmacol. 2021. PMID: 32818573 Clinical Trial. - Water extract of Ashwagandha leaves has anticancer activity: identification of an active component and its mechanism of action.
Wadhwa R, Singh R, Gao R, Shah N, Widodo N, Nakamoto T, Ishida Y, Terao K, Kaul SC. Wadhwa R, et al. PLoS One. 2013 Oct 10;8(10):e77189. doi: 10.1371/journal.pone.0077189. eCollection 2013. PLoS One. 2013. PMID: 24130852 Free PMC article. - Rational use of Ashwagandha in Ayurveda (Traditional Indian Medicine) for health and healing.
Joshi VK, Joshi A. Joshi VK, et al. J Ethnopharmacol. 2021 Aug 10;276:114101. doi: 10.1016/j.jep.2021.114101. Epub 2021 Apr 5. J Ethnopharmacol. 2021. PMID: 33831467 Review. - Effects of Withania somnifera (Ashwagandha) on Stress and the Stress- Related Neuropsychiatric Disorders Anxiety, Depression, and Insomnia.
Speers AB, Cabey KA, Soumyanath A, Wright KM. Speers AB, et al. Curr Neuropharmacol. 2021;19(9):1468-1495. doi: 10.2174/1570159X19666210712151556. Curr Neuropharmacol. 2021. PMID: 34254920 Free PMC article. Review.
Cited by
- Pistacia vera Extract Potentiates the Effect of Melatonin on Human Melatonin MT1 and MT2 Receptors with Functional Selectivity.
Labani N, Gbahou F, Noblet M, Masri B, Broussaud O, Liu J, Jockers R. Labani N, et al. Pharmaceutics. 2023 Jun 28;15(7):1845. doi: 10.3390/pharmaceutics15071845. Pharmaceutics. 2023. PMID: 37514032 Free PMC article. - Octacosanol restores stress-affected sleep in mice by alleviating stress.
Kaushik MK, Aritake K, Takeuchi A, Yanagisawa M, Urade Y. Kaushik MK, et al. Sci Rep. 2017 Aug 21;7(1):8892. doi: 10.1038/s41598-017-08874-2. Sci Rep. 2017. PMID: 28827687 Free PMC article. - Efficacy and Safety of Ashwagandha (Withania somnifera) Root Extract in Insomnia and Anxiety: A Double-blind, Randomized, Placebo-controlled Study.
Langade D, Kanchi S, Salve J, Debnath K, Ambegaokar D. Langade D, et al. Cureus. 2019 Sep 28;11(9):e5797. doi: 10.7759/cureus.5797. Cureus. 2019. PMID: 31728244 Free PMC article. - Study of Drug Target Identification and Associated Molecular Mechanisms for the Therapeutic Activity and Hair Follicle Induction of Two Ashwagandha Extracts Having Differential Withanolide Constitutions.
Cavaleri F, Chattopadhyay S, Palsule V, Kar PK, Chatterjee R. Cavaleri F, et al. J Nutr Metab. 2023 Sep 30;2023:9599744. doi: 10.1155/2023/9599744. eCollection 2023. J Nutr Metab. 2023. PMID: 37808919 Free PMC article. - Continuous intrathecal orexin delivery inhibits cataplexy in a murine model of narcolepsy.
Kaushik MK, Aritake K, Imanishi A, Kanbayashi T, Ichikawa T, Shimizu T, Urade Y, Yanagisawa M. Kaushik MK, et al. Proc Natl Acad Sci U S A. 2018 Jun 5;115(23):6046-6051. doi: 10.1073/pnas.1722686115. Epub 2018 May 21. Proc Natl Acad Sci U S A. 2018. PMID: 29784823 Free PMC article.
References
- Patchev V, Felszeghy K, Koranyi L. Neuroendocrine and neurochemical consequences of long-term sleep deprivation in rats: similarities to some features of depression. Homeost Health Dis. 1991;33(3):97–108. - PubMed
- Zakusov VV, Ostrovskaya RU, Kozhechkin SN, Markovich VV, Molodavkin GM, Voronina TA. Further evidence for GABA-ergic mechanisms in the action of benzodiazepines. Archives internationales de pharmacodynamie et de therapie. 1977;229(2):313–26. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical