A Protein Isolate from Moringa oleifera Leaves Has Hypoglycemic and Antioxidant Effects in Alloxan-Induced Diabetic Mice - PubMed (original) (raw)
A Protein Isolate from Moringa oleifera Leaves Has Hypoglycemic and Antioxidant Effects in Alloxan-Induced Diabetic Mice
Paulo C Paula et al. Molecules. 2017.
Abstract
Moringa oleifera has been used in traditional medicine to treat diabetes. However, few studies have been conducted to relate its antidiabetic properties to proteins. In this study, a leaf protein isolate was obtained from M. oleifera leaves, named _Mo_-LPI, and the hypoglycemic and antioxidant effects on alloxan-induced diabetic mice were assessed. _Mo_-LPI was obtained by aqueous extraction, ammonium sulphate precipitation and dialysis. The electrophoresis profile and proteolytic hydrolysis confirmed its protein nature. _Mo_-LPI showed hemagglutinating activity, cross-reaction with anti-insulin antibodies and precipitation after zinc addition. Single-dose intraperitoneal (i.p.) administration of _Mo_-LPI (500 mg/kg·bw) reduced the blood glucose level (reductions of 34.3%, 60.9% and 66.4% after 1, 3 and 5 h, respectively). The effect of _Mo_-LPI was also evidenced in the repeated dose test with a 56.2% reduction in the blood glucose level on the 7th day after i.p. administration. _Mo_-LPI did not stimulate insulin secretion in diabetic mice. _Mo-_LPI was also effective in reducing the oxidative stress in diabetic mice by a decrease in malondialdehyde level and increase in catalase activity. _Mo_-LPI (2500 mg/kg·bw) did not cause acute toxicity to mice. _Mo_-LPI is a promising alternative or complementary agent to treat diabetes.
Keywords: Moringa; antioxidant activity; diabetes therapy; hypoglycemic activity; plant protein.
Conflict of interest statement
The authors declare no conflict of interest. The funding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Figure 1
Assessment of the in vitro digestibility of _Mo_-LPI by SDS-PAGE analysis. _Mo_-LPI was incubated with pepsin or trypsin at different times (0, 2 and 4 h). (a) Pepsin digestion; (b) Trypsin digestion. Bovine serum albumin (BSA) was used as control. Twenty microgram from each sample were loaded in each well. Vertical numbers indicate the molecular mass markers: phosphorylase B (97 kDa); BSA (67 kDa); ovalbumin (45 kDa); carbonic anhydrase (29 kDa); trypsin inhibitor (20.1 kDa) and α-lactalbumin (14.2 kDa). Horizontal numbers refer to the digestion times (h).
Figure 1
Assessment of the in vitro digestibility of _Mo_-LPI by SDS-PAGE analysis. _Mo_-LPI was incubated with pepsin or trypsin at different times (0, 2 and 4 h). (a) Pepsin digestion; (b) Trypsin digestion. Bovine serum albumin (BSA) was used as control. Twenty microgram from each sample were loaded in each well. Vertical numbers indicate the molecular mass markers: phosphorylase B (97 kDa); BSA (67 kDa); ovalbumin (45 kDa); carbonic anhydrase (29 kDa); trypsin inhibitor (20.1 kDa) and α-lactalbumin (14.2 kDa). Horizontal numbers refer to the digestion times (h).
Figure 2
Dot blot assay using human anti-insulin as primary antibodies. (a) Tris-buffered saline; (b) Human recombinant insulin (2 mg/mL) incubated with human anti-insulin IgG (1:250); (c–e) _Mo_-LPI (2 mg/mL) incubated with primary antibodies diluted 1:250, 1:500 and 1:1000, respectively.
Figure 3
Zn-induced precipitation. (a,c) zinc-free human recombinant insulin (5 mg/mL) and _Mo_-LPI (5 mg/mL) solutions in 0.05 M Tris-HCl, pH 7.5, respectively; (b,d) zinc-free human recombinant insulin and _Mo_-LPI solutions after the addition of 1 M zinc chloride, respectively. Arrows indicate the protein precipitate.
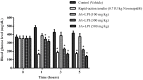
Figure 4
Effect of intraperitoneal administration of _Mo_-LPI on blood glucose level in alloxan-induced diabetic mice. Values are means ± S.E.M. (n = 10). Control: vehicle (0.05 M Tris-HCl, pH 7.5, containing 0.15 M NaCl). * Significant (p < 0.01) difference when compared with the corresponding value of the control at the same time.
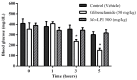
Figure 5
Influence of temperature on the hypoglycemic activity of _Mo_-LPI administered to alloxan-induced diabetic mice by intraperitoneal injection. Values are means ± S.E.M. (n = 10). Control: vehicle (0.05 M Tris-HCl, pH 7.5, containing 0.15 M NaCl). _Mo_-LPI unheated and previously boiled to 98 °C for 1 h. * Significant (p < 0.01) difference when compared with the corresponding value of the control at the same time.
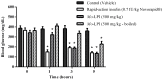
Figure 6
Effect of oral administration of _Mo_-LPI on blood glucose level in alloxan-induced diabetic mice. Values are means ± S.E.M. (n = 10). Control: vehicle (0.05 M Tris-HCl, pH 7.5, 0.15 M NaCl). * Significant (p < 0.01) difference when compared with the corresponding value of the control at the same time.
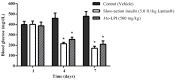
Figure 7
Effect of _Mo_-LPI on blood glucose level after seven consecutive days of intraperitoneal administration to alloxan-induced diabetic mice. Values are means ± S.E.M. (n = 10). Control: vehicle (0.05 M Tris-HCl, pH 7.5, 0.15 M NaCl). * Significant (p < 0.01) difference when compared with the corresponding value of the diabetic control.
Figure 8
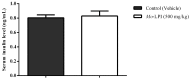
Effect of _Mo_-LPI on serum insulin level in alloxan-induced diabetic mice. Values are means ± S.E.M. (n = 10). Five hours after _Mo_-LPI or 0.15 M NaCl i.p. administration in alloxan-diabetic mice, blood samples were collected via cardiac puncture to assay the insulin level. Control: vehicle (0.05 M Tris-HCl, pH 7.5, containing 0.15 M NaCl).
Similar articles
- An aqueous extract from Moringa oleifera leaves ameliorates hepatotoxicity in alloxan-induced diabetic rats.
Abd Eldaim MA, Shaban Abd Elrasoul A, Abd Elaziz SA. Abd Eldaim MA, et al. Biochem Cell Biol. 2017 Aug;95(4):524-530. doi: 10.1139/bcb-2016-0256. Epub 2017 Apr 19. Biochem Cell Biol. 2017. PMID: 28423281 - Evaluation of Antidiabetic Effect of Combined Leaf and Seed Extracts of Moringa oleifera (Moringaceae) on Alloxan-Induced Diabetes in Mice: A Biochemical and Histological Study.
Aljazzaf B, Regeai S, Elghmasi S, Alghazir N, Balgasim A, Hdud Ismail IM, Eskandrani AA, Shamlan G, Alansari WS, Al-Farga A, Alghazeer R. Aljazzaf B, et al. Oxid Med Cell Longev. 2023 May 12;2023:9136217. doi: 10.1155/2023/9136217. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 37215365 Free PMC article. - Insulin-like plant proteins as potential innovative drugs to treat diabetes-The Moringa oleifera case study.
Paula PC, Oliveira JTA, Sousa DOB, Alves BGT, Carvalho AFU, Franco OL, Vasconcelos IM. Paula PC, et al. N Biotechnol. 2017 Oct 25;39(Pt A):99-109. doi: 10.1016/j.nbt.2016.10.005. Epub 2016 Oct 11. N Biotechnol. 2017. PMID: 27737801 Review. - Cultivation, Genetic, Ethnopharmacology, Phytochemistry and Pharmacology of Moringa oleifera Leaves: An Overview.
Leone A, Spada A, Battezzati A, Schiraldi A, Aristil J, Bertoli S. Leone A, et al. Int J Mol Sci. 2015 Jun 5;16(6):12791-835. doi: 10.3390/ijms160612791. Int J Mol Sci. 2015. PMID: 26057747 Free PMC article. Review.
Cited by
- Optimizing an Enzymatic Extraction Method for the Flavonoids in Moringa (Moringa oleifera Lam.) Leaves Based on Experimental Designs Methodologies.
Polo-Castellano C, Mateos RM, Visiedo F, Palma M, Barbero GF, Ferreiro-González M. Polo-Castellano C, et al. Antioxidants (Basel). 2023 Feb 3;12(2):369. doi: 10.3390/antiox12020369. Antioxidants (Basel). 2023. PMID: 36829929 Free PMC article. - Moringa Oleifera Lam. in Cardiometabolic Disorders: A Systematic Review of Recent Studies and Possible Mechanism of Actions.
Louisa M, Patintingan CGH, Wardhani BWK. Louisa M, et al. Front Pharmacol. 2022 Mar 30;13:792794. doi: 10.3389/fphar.2022.792794. eCollection 2022. Front Pharmacol. 2022. PMID: 35431967 Free PMC article. - Moringa Genus: A Review of Phytochemistry and Pharmacology.
Abd Rani NZ, Husain K, Kumolosasi E. Abd Rani NZ, et al. Front Pharmacol. 2018 Feb 16;9:108. doi: 10.3389/fphar.2018.00108. eCollection 2018. Front Pharmacol. 2018. PMID: 29503616 Free PMC article. Review. - Promising Influences of Moringa oleifera in Functional Foods against Metabolic Syndrome: A Comprehensive and Mechanistic Review.
Masoumvand M, Ramezani E, Rahimi VB, Askari VR. Masoumvand M, et al. Endocr Metab Immune Disord Drug Targets. 2024;24(12):1355-1370. doi: 10.2174/0118715303269893231207071440. Endocr Metab Immune Disord Drug Targets. 2024. PMID: 38279759 Review. - Effects of Moringa oleifera on Glycaemia and Insulin Levels: A Review of Animal and Human Studies.
Vargas-Sánchez K, Garay-Jaramillo E, González-Reyes RE. Vargas-Sánchez K, et al. Nutrients. 2019 Dec 2;11(12):2907. doi: 10.3390/nu11122907. Nutrients. 2019. PMID: 31810205 Free PMC article. Review.
References
- American Diabetes Association Diagnosis and classification of diabetes. Diabetes Care. 2016;39:S13–S22. - PubMed
- Xavier-Filho J., Oliveira A.E.A., Silva L.B., Azevedo C.R., Venâncio T.M., Machado O.L.T., Oliva M.L., Fernandes K.V.S., Xavier-Neto J. Plant insulin or glucokinin: A conflicting issue. Braz. J. Plant Physiol. 2003;15:67–78. doi: 10.1590/S1677-04202003000200002. - DOI
- Mentreddy S.R. Medicinal plant species with potential antidiabetic properties. J. Sci. Food Agric. 2007;87:743–750. doi: 10.1002/jsfa.2811. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical