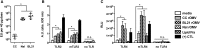Safe Recombinant Outer Membrane Vesicles that Display M2e Elicit Heterologous Influenza Protection - PubMed (original) (raw)
. 2017 Apr 5;25(4):989-1002.
doi: 10.1016/j.ymthe.2017.01.010. Epub 2017 Feb 16.
C Garrett Rappazzo 1, Jaclyn S Higgins 2, Xiangjie Sun 3, Nicole Brock 3, Annie Chau 2, Aditya Misra 4, Joseph P B Cannizzo 5, Michael R King 1, Taronna R Maines 3, Cynthia A Leifer 6, Gary R Whittaker 6, Matthew P DeLisa 4, David Putnam 7
Affiliations
- PMID: 28215994
- PMCID: PMC5383554
- DOI: 10.1016/j.ymthe.2017.01.010
Safe Recombinant Outer Membrane Vesicles that Display M2e Elicit Heterologous Influenza Protection
Hannah C Watkins et al. Mol Ther. 2017.
Abstract
Recombinant, Escherichia coli-derived outer membrane vesicles (rOMVs), which display heterologous protein subunits, have potential as a vaccine adjuvant platform. One drawback to rOMVs is their lipopolysaccharide (LPS) content, limiting their translatability to the clinic due to potential adverse effects. Here, we explore a unique rOMV construct with structurally remodeled lipids containing only the lipid IVa portion of LPS, which does not stimulate human TLR4. The rOMVs are derived from a genetically engineered B strain of E. coli, ClearColi, which produces lipid IVa, and which was further engineered in our laboratory to hypervesiculate and make rOMVs. We report that rOMVs derived from this lipid IVa strain have substantially attenuated pyrogenicity yet retain high levels of immunogenicity, promote dendritic cell maturation, and generate a balanced Th1/Th2 humoral response. Additionally, an influenza A virus matrix 2 protein-based antigen displayed on these rOMVs resulted in 100% survival against a lethal challenge with two influenza A virus strains (H1N1 and H3N2) in mice with different genetic backgrounds (BALB/c, C57BL/6, and DBA/2J). Additionally, a two-log reduction of lung viral titer was achieved in a ferret model of influenza infection with human pandemic H1N1. The rOMVs reported herein represent a potentially safe and simple subunit vaccine delivery platform.
Keywords: M2e; adjuvants; endotoxin; influenza; outer membrane vesicles; subunit vaccine delivery.
Copyright © 2017 The American Society of Gene and Cell Therapy. All rights reserved.
Figures
Graphical abstract
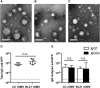
Figure 1
rOMVs Produced from Three Different E. coli Strains Are Structurally Comparable (A–C) TEM images of rOMVs stained with uranyl acetate: CC rOMVs (A), BL21 rOMVs (B), and Nsl rOMVs (C). Scale bars represent 100 nm. (D and E) Total IgG (D) and isotypes IgG1 and IgG2a (E) anti-GFP titers from BALB/c mice 8 weeks post prime dose of ClyA-GFP-expressing CC or BL21 rOMVs. Titer error bars represent 95% confidence intervals (CI) of geometric mean. Log-transformed data analyzed using an unpaired Student’s t test to compare CC versus BL21 rOMV anti-GFP IgG levels and using paired Student’s t test to compare IgG1:IgG2a levels for each rOMV type. Dotted line indicates titer of sera from mice pre-vaccination.
Figure 2
Pyrogenicity and TLR Activity Are rOMV Source Strain Dependent (A) Pyrogenicity (measured in endotoxin units) of CC, Nsl, and BL21 rOMVS determined using whole-blood pyrogenicity test. Groups were compared with Kruskal-Wallis test, followed by Mann-Whitney between pairs, using Bonferroni method to account for multiple comparisons (*p < 0.01). Error bars represent 95% CI of geometric mean (n = 5 blood donors). (B) HEK-Blue KD-TLR5 cells transfected with human TLR2 or mTLR2 were stimulated with rOMVs (100 ng/mL) or Pam3Cys (1 μg/mL, (+) CTL) for 16 hr. (C) HEK-Blue KD-TLR5 cells transfected with 5x NF-κB-luciferase reporter and TLR4/MD-2/CD14 or mTLR4/mMD-2/mCD14 were stimulated with rOMVs (100 ng/mL), lipid IVa (100 ng/mL), or LPS (100 ng/mL, (+) CTL) for 16 hr. Samples analyzed by ANOVA followed by multiple comparisons against media using Dunnett method of correction (*p < 0.0001). Error bars represent SD (n = 4).
Figure 3
Bone Marrow-Derived Dendritic Cells Are Activated by rOMVs Murine BMDCs from BALB/c and C57BL/6 mice were stimulated with Nsl rOMVs (100 ng/mL), CC rOMVs (100 ng/mL), LPS (100 ng/mL), or PBS for 24 or 48 hr, then supernatants were collected for cytokine analysis, and cells were stained for DC maturation markers (stains: viability, CD11c, CD86, MHCII). Flow cytometry was used to determine percent mature dendritic cells (DCs) (CD86Hi, MHCIIHi) out of the total DC population (gated on viability, CD11cHi). (A) Representative density plots of BMDCs isolated from a BALB/c mouse. (B) Percent mature DCs from BALB/c mice. (C) Percent mature DCs from C57BL/6 mice. (D) IL-10 concentration after 24-hr stimulation. (E) IL-12p70 concentration (conc.) after 24-hr stimulation. (F) Type 1 IFN conc. after 24-hr stimulation. (G) IL-6 conc. after 24-hr stimulation. (H) TNF-α conc. after 24-hr stimulation. Cytokine stimulation is shown for both C57BL/6 and BALB/c mice. Error bars represent SD. Samples analyzed via ANOVA followed by Holm multiple-comparison test, *p < 0.05 (n = 3 mice).
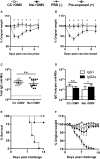
Figure 4
rOMV Source Strain Dependence on Morbidity, Immune Response, and Mortality (A and B) Mice were weighed daily for 1 week post-prime (A) and boost (B) immunization. Analyzed using ANOVA followed by multiple comparisons using Dunnett method of correction. Error bars represent SD of mean (*p < 0.001). (C and D) Total IgG (C) and IgG isotypes IgG1 and IgG2a (D) anti-M2e titers of BALB/c mice 8 weeks post-prime rOMV immunization. Dotted line indicates lowest titer detectable above background (serum from PBS-vaccinated mice). Log-transformed IgG1 and IgG2a anti-M2e titers compared using paired t test. Error bars indicate 95% CI of geometric mean (*p < 0.05) (n = 11 CC rOMV-vaccinated mice, n = 12 Nsl rOMV-vaccinated mice, n = 16 PBS-vaccinated mice). (E and F) Mortality (E) and weight loss (F) of mice challenged with a lethal dose (50 FFU) of influenza A/PR8 (n = 5 CC rOMV vaccinated, n = 5 Nsl rOMV vaccinated, n = 5 PBS vaccinated, n = 5 pre-exposed). Kaplan-Meier survival curves were analyzed with a log-rank test using the Bonferroni method to account for multiple comparisons. Error bars on morbidity curves represent SEM.
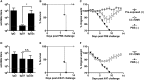
Figure 5
Immune Response, Mortality, and Morbidity in C57BL/6 and DBA/2J Mice (A) Total IgG and isotypes IgG1 and IgG2c titers of C57BL/6 mice 8 weeks post-prime rOMV vaccination. Dotted line indicates lowest titer detectable above background. Log-transformed IgG1 and IgG2c titers compared using paired t test. Error bars indicate 95% CI of geometric mean (n = 10 CC rOMV-vaccinated mice) (*p < 0.0001). (B and C) Mortality (B) and weight loss (C) of mice challenged with a lethal dose (100 FFU) of influenza A/PR8 (n = 5 CC rOMV vaccinated, n = 5 PBS vaccinated, n = 5 pre-exposed). Kaplan-Meier survival curves were analyzed with a log-rank test. Error bars on morbidity curves represent SEM. (D) Total IgG and isotypes IgG1 and IgG2a titers of DBA/2J mice 8 weeks post-prime rOMV vaccination. Dotted line indicates lowest titer detectable above background. Log-transformed IgG1 and IgG2c titers compared using paired t test. Error bars indicate 95% CI of geometric mean (n = 5 CC rOMV-vaccinated mice) (*p < 0.0001). (E and F) Mortality (E) and morbidity (F) of DBA/2J mice challenged with a lethal dose (5,000 PFU, ∼2.5 × LD50) of influenza A/X-47 (n = 5 CC rOMV vaccinated, n = 5 PBS vaccinated). Kaplan-Meier survival curves were analyzed with a log-rank test. Error bars on morbidity curves represent SEM.
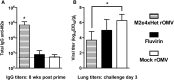
Figure 6
Ferret Antibody Titers and Lung Viral Titer Resulting from ClyA-M2e4xHet rOMVs Immunization (A) Total IgG anti-M2e titers of ferrets 8 weeks post-prime vaccination. Error bars indicate 95% CI of geometric mean (n = 6 ferrets per group) (*p < 0.01). (B) Lung viral titers of ferrets 3 days post-challenge with influenza strain pdmH1N1 (n = 3 ferrets per group). Lung titers compared using ANOVA followed by comparison to mock CC rOMVs using Bonferroni method of correction (n = 3). Error bars indicate SD of log-transformed egg infectious dose (EID50) per gram of lung tissue (*p < 0.01).
Similar articles
- Recombinant M2e outer membrane vesicle vaccines protect against lethal influenza A challenge in BALB/c mice.
Rappazzo CG, Watkins HC, Guarino CM, Chau A, Lopez JL, DeLisa MP, Leifer CA, Whittaker GR, Putnam D. Rappazzo CG, et al. Vaccine. 2016 Mar 4;34(10):1252-8. doi: 10.1016/j.vaccine.2016.01.028. Epub 2016 Jan 29. Vaccine. 2016. PMID: 26827663 - A single dose and long lasting vaccine against pandemic influenza through the controlled release of a heterospecies tandem M2 sequence embedded within detoxified bacterial outer membrane vesicles.
Watkins HC, Pagan CL, Childs HR, Posada S, Chau A, Rios J, Guarino C, DeLisa MP, Whittaker GR, Putnam D. Watkins HC, et al. Vaccine. 2017 Sep 25;35(40):5373-5380. doi: 10.1016/j.vaccine.2017.08.013. Epub 2017 Aug 31. Vaccine. 2017. PMID: 28866291 - In contrast to conventional inactivated influenza vaccines, 4xM2e.HSP70c fusion protein fully protected mice against lethal dose of H1, H3 and H9 influenza A isolates circulating in Iran.
Ebrahimi SM, Dabaghian M, Tebianian M, Jazi MH. Ebrahimi SM, et al. Virology. 2012 Aug 15;430(1):63-72. doi: 10.1016/j.virol.2012.04.015. Epub 2012 May 16. Virology. 2012. PMID: 22595444 - Development of a candidate influenza vaccine based on virus-like particles displaying influenza M2e peptide into the immunodominant region of hepatitis B core antigen: Broad protective efficacy of particles carrying four copies of M2e.
Tsybalova LM, Stepanova LA, Kuprianov VV, Blokhina EA, Potapchuk MV, Korotkov AV, Gorshkov AN, Kasyanenko MA, Ravin NV, Kiselev OI. Tsybalova LM, et al. Vaccine. 2015 Jun 26;33(29):3398-406. doi: 10.1016/j.vaccine.2015.04.073. Epub 2015 May 11. Vaccine. 2015. PMID: 25976545 - Protection against multiple influenza A virus strains induced by candidate recombinant vaccine based on heterologous M2e peptides linked to flagellin.
Stepanova LA, Kotlyarov RY, Kovaleva AA, Potapchuk MV, Korotkov AV, Sergeeva MV, Kasianenko MA, Kuprianov VV, Ravin NV, Tsybalova LM, Skryabin KG, Kiselev OI. Stepanova LA, et al. PLoS One. 2015 Mar 23;10(3):e0119520. doi: 10.1371/journal.pone.0119520. eCollection 2015. PLoS One. 2015. PMID: 25799221 Free PMC article.
Cited by
- Exosomes: Biological Pharmaceutical Nanovectors for Theranostics.
Thomas SC, Kim JW, Pauletti GM, Hassett DJ, Kotagiri N. Thomas SC, et al. Front Bioeng Biotechnol. 2022 Jan 12;9:808614. doi: 10.3389/fbioe.2021.808614. eCollection 2021. Front Bioeng Biotechnol. 2022. PMID: 35096795 Free PMC article. Review. - GMMA-Based Vaccines: The Known and The Unknown.
Mancini F, Micoli F, Necchi F, Pizza M, Berlanda Scorza F, Rossi O. Mancini F, et al. Front Immunol. 2021 Aug 3;12:715393. doi: 10.3389/fimmu.2021.715393. eCollection 2021. Front Immunol. 2021. PMID: 34413858 Free PMC article. Review. - Human gut microbiota stimulate defined innate immune responses that vary from phylum to strain.
Spindler MP, Siu S, Mogno I, Li Z, Yang C, Mehandru S, Britton GJ, Faith JJ. Spindler MP, et al. Cell Host Microbe. 2022 Oct 12;30(10):1481-1498.e5. doi: 10.1016/j.chom.2022.08.009. Epub 2022 Sep 12. Cell Host Microbe. 2022. PMID: 36099923 Free PMC article. - In Silico Design of CT26 Polytope and its Surface Display by ClearColi™-Derived Outer Membrane Vesicles as a Cancer Vaccine Candidate Against Colon Carcinoma.
Sharif E, Nezafat N, Ahmadi FM, Mohit E. Sharif E, et al. Appl Biochem Biotechnol. 2024 Dec;196(12):8820-8847. doi: 10.1007/s12010-024-04971-x. Epub 2024 Jul 3. Appl Biochem Biotechnol. 2024. PMID: 38958886 - Genetically engineered cellular nanoparticles for biomedical applications.
Krishnan N, Peng FX, Mohapatra A, Fang RH, Zhang L. Krishnan N, et al. Biomaterials. 2023 May;296:122065. doi: 10.1016/j.biomaterials.2023.122065. Epub 2023 Feb 20. Biomaterials. 2023. PMID: 36841215 Free PMC article. Review.
References
- Reed S.G., Orr M.T., Fox C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19:1597–1608. - PubMed
- Kuehn M.J., Kesty N.C. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005;19:2645–2655. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical