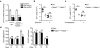Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism - PubMed (original) (raw)
. 2017 Mar 9;543(7644):252-256.
doi: 10.1038/nature21379. Epub 2017 Feb 20.
Tian Tian 1, Chang Ook Park 1, Serena Y Lofftus 1, Shenglin Mei 2, Xing Liu 3, Chi Luo 4, John T O'Malley 1, Ahmed Gehad 1, Jessica E Teague 1, Sherrie J Divito 1, Robert Fuhlbrigge 1, Pere Puigserver 4, James G Krueger 5, Gökhan S Hotamisligil 6, Rachael A Clark 1 7, Thomas S Kupper 1 7
Affiliations
- PMID: 28219080
- PMCID: PMC5509051
- DOI: 10.1038/nature21379
Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism
Youdong Pan et al. Nature. 2017.
Abstract
Tissue-resident memory T (TRM) cells persist indefinitely in epithelial barrier tissues and protect the host against pathogens. However, the biological pathways that enable the long-term survival of TRM cells are obscure. Here we show that mouse CD8+ TRM cells generated by viral infection of the skin differentially express high levels of several molecules that mediate lipid uptake and intracellular transport, including fatty-acid-binding proteins 4 and 5 (FABP4 and FABP5). We further show that T-cell-specific deficiency of Fabp4 and Fabp5 (Fabp4/Fabp5) impairs exogenous free fatty acid (FFA) uptake by CD8+ TRM cells and greatly reduces their long-term survival in vivo, while having no effect on the survival of central memory T (TCM) cells in lymph nodes. In vitro, CD8+ TRM cells, but not CD8+ TCM cells, demonstrated increased mitochondrial oxidative metabolism in the presence of exogenous FFAs; this increase was not seen in Fabp4/Fabp5 double-knockout CD8+ TRM cells. The persistence of CD8+ TRM cells in the skin was strongly diminished by inhibition of mitochondrial FFA β-oxidation in vivo. Moreover, skin CD8+ TRM cells that lacked Fabp4/Fabp5 were less effective at protecting mice from cutaneous viral infection, and lung Fabp4/Fabp5 double-knockout CD8+ TRM cells generated by skin vaccinia virus (VACV) infection were less effective at protecting mice from a lethal pulmonary challenge with VACV. Consistent with the mouse data, increased FABP4 and FABP5 expression and enhanced extracellular FFA uptake were also demonstrated in human CD8+ TRM cells in normal and psoriatic skin. These results suggest that FABP4 and FABP5 have a critical role in the maintenance, longevity and function of CD8+ TRM cells, and suggest that CD8+ TRM cells use exogenous FFAs and their oxidative metabolism to persist in tissue and to mediate protective immunity.
Conflict of interest statement
The authors declare no competing financial interests. Readers are welcome to comment on the online version of the paper.
Figures
Extended Data Figure 1. CD8+ TRM cells show increased gene expression of Fabp4 and Fabp5
a, Number of OT-I Thy1.1+ cells in infected skin at indicated time after infection. Graph shows mean ± s.d. of 10 mice. b, Venn diagram analysis of genes differentially expressed in pairwise comparisons between OT-I TCM, TEM and TRM cells (day 30) relative to that of TN cells (fold change cutoff, ≥ 2). c, Absolute qPCR analysis of Fabp gene expression in TRM cells (day 30). ND, not detectable. d, qPCR assessments of Fabp4 and Fabp5 levels in indicated T cell subsets. OT-I Thy1.1+ cells were intravenously transferred into Thy1.2+ recipient mice 1 day before mice were infected with 2 × 106 p.f.u. VACVOVA by intratracheal infection. After 45 days, mice were euthanized. TCM and TEM cells were sorted from spleen and TRM cells were sorted from lung. e, qPCR analysis of _Pparg_-knockdown efficiency by lentiviral vector encoding two specific and one scrambled siRNA in OT-I CD8+ TRM cells. f, qPCR analysis of Fabp4 and Fabp5 gene expression in OT-I CD8+ TRM cells from mice treated with or without GW9662. Graphs in c, d, e, f show mean ± s.d. from triplicates. β-actin was used as internal control and mRNA was normalized to TN samples (d), or TRM samples with scrambled siRNA transduction (e) or without GW9662 treatment (f). T cells from 15–20 mice were pooled for each sample. **P < 0.01; NS, not significant.
Extended Data Figure 2. Visualization of lipid distribution in mouse skin tissue by Bodipy staining
Mouse skin cryosections were stained for lipids (4,4-Difluoro-1,3,5,7,8-Pentamethyl-4-Bora-3a,4a-Diaza-s-Indacene (BODIPY 493/503), green) and nuclei (4′,6-diamidino-2-phenylindole (DAPI), blue). Images were acquired with a Leica TCS SP8 confocal microscope and analysed with ImageJ. n = 15 sections from 5 mice and 1 representative site is shown. Dashed line indicates epidermal–dermal boundary. Scale bar, 20 μm.
Extended Data Figure 3. OT-I _Fabp4_−/−/_Fabp5_−/− TRM cells show a similar surface protein expression phenotype as wild-type cells
Thy1.2+ CD45.2+ recipient mice were given a 1:1 ratio of naive OT-I Thy1.1+ CD45.1+ wild-type and OT-I Thy1.1+ CD45.2+ _Fabp4_−/−/_Fabp5_−/− cells one day before mice were infected with 2 × 106 p.f.u. VACVOVA by skin scarification. Subsequently, 45 days after infection, infected skin sites were collected and the phenotype of TRM cells was examined by flow cytometry. Data are representative of three independent experiments (n = 5 mice per group). ESL, E-selectin ligand.
Extended Data Figure 4. Relative contribution of FABP4 and FABP5 in fatty acid acquisition and long-term maintenance of skin CD8+ TRM cells
a, Average MFI of Bodipy FL C16 uptake by OT-I wild-type, Fabp4+/−Fabp5+/−, _Fabp4_−/− and Fabp5_−/− TRM cells. b–d, Number of OT-I wild-type, Fabp4+/−_Fabp5+/−, _Fabp4_−/− and Fabp5_−/− TRM cells at different time points after VACVOVA infection. Thy1.2+ CD45.2+ recipient mice were given a 1:1 ratio of naive OT-I Thy1.1+ CD45.1+ wild-type and OT-I Thy1.1+ CD45.2+ Fabp4/Fabp5 knockout cells (Fabp4+/−_Fabp5+/− (b), _Fabp4_−/− (c) or _Fabp5_−/− (d) cells) one day before infection with 2 × 106 p.f.u. VACVOVA by skin scarification. Relative percentages of the two T cell populations were analysed longitudinally by flow cytometry. Graphs show mean ± s.d. of 5 mice per group. NS, not significant.
Extended Data Figure 5. OT-I _Fabp4_−/−/_Fabp5_−/− Teff cells have similar proliferative capacity and tissue-homing receptor expression as wild-type counterparts
a, Quantification of Ki67+ cells in OT-I TN, Teff, TCM, TEM and TRM cells. b, Average MFI of Bodipy FL C16 uptake by OT-I TN, Teff, TCM, TEM or TRM cells. Graphs show mean ± s.d. of 5 mice per group (a, b). *P < 0.05; **P < 0.01. c, Flow cytometric analysis of T cell proliferation and homing receptor expression on OT-I wild-type and _Fabp4_−/−/_Fabp5_−/− T cells. Thy1.2+ CD45.2+ recipient mice were given a 1:1 ratio of CFSE-labelled naive OT-I Thy1.1+ CD45.1+ wild-type and OT-I Thy1.1+ CD45.2+ _Fabp4_−/−/_Fabp5_−/− cells one day before mice were infected with 2 × 106 p.f.u. VACVOVA by skin scarification. At 60 h after infection, proliferation and tissue-homing receptor expression of OT-I cells isolated from draining lymph nodes were analysed by flow cytometry. Data are representative of three independent experiments (n = 5 mice per group). ESL, E-selectin ligand; PSL, P-selectin ligand.
Extended Data Figure 6. Effect of Pparg lentiviral siRNA knockdown or PPARγ inhibition on CD8+ TRM cell maintenance in peripheral tissue
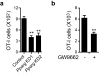
a, Number of OT-I CD8+ TRM transduced with scrambled siRNA or siRNA targeting Pparg. OT-I cells transduced with Pparg siRNA (together with the same number of congenically scrambled siRNA transduced OT-I cells) were cotransferred into recipient mice that were previously infected with VACVOVA by skin scarification. After 40 days, mice were euthanized and the number of siRNA-transduced OT-I cells in the infected skin tissue were collected and counted by flow cytometry on the basis of the GFP marker. b, Number of OT-I CD8+ TRM cells in infected skin from mice treated with or without GW9662. Thy1.2+ CD45.2+ recipient mice were infected with 2 × 106 p.f.u. VACVOVA by skin scarification. 40 days later, mice were treated with GW9662 daily by intradermal injection for 5 days, after which mice were euthanized and TRM cells were counted and analysed by flow cytometry. Graphs show mean ± s.d. of 5 mice per group. **P < 0.01.
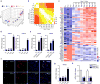
Extended Data Figure 7. Gene expression profile of OT-I _Fabp4_−/−/_Fabp5_−/− TRM cells
a, Principal-component analysis of gene-expression data for skin infiltrating T cells isolated at day 10 and day 30 after infection. b, Differentially expressed genes selected from a pairwise comparison between OT-I _Fabp4_−/−/_Fabp5_−/− TRM and OT-I wild-type TRM cells. Thy1.2+ CD45.2+ recipient mice were given a 1:1 ratio of naive OT-I Thy1.1+ CD45.1+ wild-type and OT-I Thy1.1+ CD45.2+ _Fabp4_−/−/_Fabp5_−/− cells one day before mice were infected with 2 × 106 p.f.u. VACVOVA by skin scarification. At 30 days after infection, OT-I CD8+ TRM cells were sorted from infected skin sites for gene microarray. c, qPCR analysis of genes involved in anti-inflammatory responses (Il10 and Socs2), cell apoptosis (Casp3 and Bcl2113) as well as immune responses (Ccr8, Ccl21, Il1r1, Il6, Cxcl13, Cxcl12, Ccl12, Gzmc, Gzma) in OT-I _Fabp4_−/−/_Fabp5_−/− TRM cells compared to wild-type TRM cells. Graphs show mean ± s. d. from triplicates. β-actin was used as internal control and mRNA was normalized to OT-I wild-type TRM cells. mRNA was pooled from 15 mice from 3 independent biological groups (5 mice per group). **P < 0.01.
Extended Data Figure 8. Effect of Cpt1a lentiviral siRNA knockdown or CPT1A inhibition on OT-I CD8+ TRM cell maintenance in peripheral tissue
a, qPCR analysis of Cpt1a lentiviral siRNA knockdown efficiency in OT-I CD8+ TRM cells. b, c, Quantification of OT-I wild-type and OT-I _Fabp4_−/−/_Fabp5_−/− TRM cells in infected skin from mice treated with or without etomoxir (b) or trimetazidine (c). Thy1.2+ CD45.2+ recipient mice were given a 1:1 ratio of naive OT-I Thy1.1+ CD45.1+ wild-type and OT-I Thy1.1+ CD45.2+ _Fabp4_−/−/_Fabp5_−/− cells one day before infection with 2 × 106 p.f.u. VACVOVA by skin scarification. Mice were administered etomoxir or trimetazidine daily by intradermal injection for 5 days from day 40 after infection, after which mice were euthanized and TRM cells were analysed by flow cytometry and counted. Symbols represent individual mice. d, OCR and ECAR as measured by the Seahorse assay of skin-infiltrating T cells sorted at indicated time points after infection under basal conditions. Graphs show mean ± s.d. of triplicates. **P < 0.01; NS, not significant.
Extended Data Figure 9. Skin CD8+ TRM cells residing in distal infection sites are deficient in long-time survival and virus clearance
a, Ratio of peripheral blood CD3 counts in FTY720 versus untreated mice, illustrating the kinetics of lymphoid sequestration by FTY720. n = 5 mice per group. b, Quantification of OT-I wild-type and OT-I _Fabp4_−/−/_Fabp5_−/− cells infiltrating distal infection sites at different time points after VACVOVA infection. Thy1.2+ CD45.2+ recipient mice were given a 1:1 ratio of naive OT-I Thy1.1+ CD45.1+ wild-type and OT-I Thy1.1+ CD45.2+ _Fabp4_−/−/_Fabp5_−/− cells one day before infection with 2 × 106 p.f.u. VACVOVA by skin scarification of the right ears. Relative percentages of the two T cell populations infiltrating left ears were analysed longitudinally by flow cytometry. Graphs show mean ± s.d. of 5 mice per group. c, qPCR analysis of distal infection skin viral load at six days after re-challenge. OT-I wild-type or OT-I _Fabp4_−/−/_Fabp5_−/− cells were adoptively transferred into μMT mice before infection with 2 × 106 p.f.u. VACVOVA by skin scarification of the right ears. 25 days later, mice were re-challenged with VACVOVA at the left ear by skin scarification. U, unimmunized; I, immunized mice. Symbols represent individual mice. **P < 0.01; NS, not significant.
Extended Data Figure 10. Visualization of lipid distribution in human skin from patients with psoriasis by Bodipy staining
Cryosections of lesional scalp skin samples from patients with psoriasis were stained for lipid (4,4-Difluoro-1,3,5,7,8-Pentamethyl-4-Bora-3a,4a-Diaza-s-Indacene (BODIPY 493/503), green) and nuclei (4′,6-diamidino-2-phenylindole (DAPI), blue). Images were acquired with a Leica TCS SP8 confocal microscope and analysed with ImageJ. Dashed lines indicate epidermal–dermal boundary. Scale bar, 50 μm.
Figure 1. Skin CD8+ TRM cells show increased expression of FABP4 and FABP5
a, Principal-component analysis (PCA) of gene-expression data for CD8+ T cell subtypes. Each time point represents an individual experiment wherein mRNA was pooled from 15–20 mice from 3–4 independent biological groups (5 mice per group). Numbered dots are for skin T cells derived after infection for the indicated number of days. b, Pearson correlation coefficients among CD8+ T cell subtypes. c, Heatmap of differentially expressed genes selected from a pair-wise comparison between OT-I TRM (day 30) and TCM cells. d, qPCR analysis of Fabp4 and Fabp5 expression in TN, TCM, TEM and TRM cells (day 30). e, qPCR analysis of Fabp4 and Fabp5 gene expression in skin CD103− and CD103+ TRM cells (day 30). f, Immunofluorescence staining of FABP4 (top) and FABP5 (bottom) in OT-I TRM cells 30 days after infection. Scale bar, 20 μm. g, qPCR analysis of Pparg expression in TN, TCM, TEM and TRM (day 30). h, Effect of lentiviral Pparg siRNA knockdown (KD) on Fabp4 and Fabp5 expression in OT-I CD8+ TRM cells. Graphs in d, e, g, h show mean ± s.d. from triplicates. β-actin was used as internal control and mRNA was normalized to TN cells (d, e, g) or TRM cells transduced with a lentiviral vector encoding scrambled siRNA (h). T cells from 15–20 mice were pooled for each group. **P < 0.01; NS, not significant.
Figure 2. Loss of Fabp4 and Fabp5 decreases fatty-acid uptake and metabolism by CD8+ TRM cells and impairs their long-term maintenance
a, Representative histograms and average mean fluourescence intensity (MFI) of Bodipy FL C16 uptake by TN, TCM, TEM or TRM cells (day 30). b, Representative histograms and average MFI of Bodipy FL C16 uptake in OT-I wild-type and _Fabp4_−/−/_Fabp5_−/− TRM cells with or without pre-incubation with unlabelled palmitate (Palm). c, Number of OT-I wild-type and _Fabp4_−/−/_Fabp5_−/− TCM and TRM cells at different time points after infection in spleen and skin. d, Immunofluorescence staining and quantification of OT-I wild-type and _Fabp4_−/−/_Fabp5_−/− TRM cells at infected skin sites 45 days after infection. FOV, field of view. Scale bar, 50 μm. e, Representative histograms and quantification of annexin V+ cells in OT-I wild-type and _Fabp4_−/−/_Fabp5_−/− TRM cells 45 days after infection. f, OCR of OT-I TCM, TRM and _Fabp4_−/−/_Fabp5_−/− TRM cells (day 30) under basal conditions and in response to indicated mitochondria inhibitors. Results were normalized to control cells treated with bovine serum albumin (BSA). Eto, etomoxir. Graphs show mean ± s.d. of triplicates. g, Effect of lentiviral Cpt1a siRNA knockdown on OT-I CD8+ TRM survival in vivo in infected tissue. h, Representative dot plots and percentage of wild-type and _Fabp4_−/−/_Fabp5_−/− TCM and TRM cells 45 days after infection. Cells were gated on VACV-specific pentamer+ CD8+ T cells. Graphs show mean ± s.d. of 5 (a, b) or 10 (c) mice, or 50 fields from 5 mice (d, 10 fields per mouse), or symbols represent individual mice (e, g, h). *P <0.05; **P < 0.01; NS, not significant.
Figure 3. Skin _Fabp4_−/−/_Fabp5_−/− CD8+ TRM cells fail to protect mice against viral infectious challenge
a, Schematic of experimental design. i.p., intraperitoneal injection; s.s., b, qPCR analysis of skin viral load at six days after re-infection. U, un-immunized mice; I, immunized mice. c, d, Representative dot plots and quantification of IFNγ secretion by OT-I wild-type or _Fabp4_−/−/_Fabp5_−/− TRM cells. Symbols represent individual mice (b, d). e, Schematic of experimental design. f, g, Body weight (BW) (f) and survival measurements (g) of WR-VACV challenged mice, with or without FTY720 treatment. Data are representative of three independent experiments (n = 10 mice per group). *P < 0.05; **P < 0.01; NS, not significant.
Figure 4. Human skin CD8+ TRM demonstrate increased expression of FABP4 and FABP5
a, b, Representative histograms and average MFI of intracellular staining of FABP4 (a) and FABP5 (b) in human peripheral blood mononuclear TN, TCM, and TEM cells compared to facial skin TRM. c, Haematoxylin and eosin staining of a human psoriatic lesion. Scale bar, 100 μm. d, Immunofluorescence staining of CD8 and CD69 expression in psoriatic human skin. Dashed line indicates epidermal–dermal junction. Scale bar, 50 μm. e, Immunofluorescence staining of FABP4 (top) and FABP5 (bottom) expression in skin CD8+ TRM cells from patients with psoriasis. Scale bar, 20 μm. f, Representative histograms and average MFI of Bodipy FL C16 uptake by human TN, TCM, TEM or TRM cell subsets. a, b, f, Graphs show mean ± s.d. of 5 individuals; c–e, Representative images of n = 15 sections from 5 individuals. **P < 0.01.
Comment in
- Tissue-resident memory T cells live off the fat of the land.
Stolley JM, Masopust D. Stolley JM, et al. Cell Res. 2017 Jul;27(7):847-848. doi: 10.1038/cr.2017.49. Epub 2017 Mar 31. Cell Res. 2017. PMID: 28361896 Free PMC article.
Similar articles
- Fatty Acid Oxidation Controls CD8+ Tissue-Resident Memory T-cell Survival in Gastric Adenocarcinoma.
Lin R, Zhang H, Yuan Y, He Q, Zhou J, Li S, Sun Y, Li DY, Qiu HB, Wang W, Zhuang Z, Chen B, Huang Y, Liu C, Wang Y, Cai S, Ke Z, He W. Lin R, et al. Cancer Immunol Res. 2020 Apr;8(4):479-492. doi: 10.1158/2326-6066.CIR-19-0702. Epub 2020 Feb 19. Cancer Immunol Res. 2020. PMID: 32075801 - Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity.
Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Jiang X, et al. Nature. 2012 Feb 29;483(7388):227-31. doi: 10.1038/nature10851. Nature. 2012. PMID: 22388819 Free PMC article. - Dual role of fatty acid-binding protein 5 on endothelial cell fate: a potential link between lipid metabolism and angiogenic responses.
Yu CW, Liang X, Lipsky S, Karaaslan C, Kozakewich H, Hotamisligil GS, Bischoff J, Cataltepe S. Yu CW, et al. Angiogenesis. 2016 Jan;19(1):95-106. doi: 10.1007/s10456-015-9491-4. Epub 2015 Dec 1. Angiogenesis. 2016. PMID: 26625874 Free PMC article. - Pathophysiology of Skin Resident Memory T Cells.
Tokura Y, Phadungsaksawasdi P, Kurihara K, Fujiyama T, Honda T. Tokura Y, et al. Front Immunol. 2021 Feb 3;11:618897. doi: 10.3389/fimmu.2020.618897. eCollection 2020. Front Immunol. 2021. PMID: 33633737 Free PMC article. Review. - CD8+ Resident Memory T Cells and Viral Infection.
Wu X, Wu P, Shen Y, Jiang X, Xu F. Wu X, et al. Front Immunol. 2018 Sep 19;9:2093. doi: 10.3389/fimmu.2018.02093. eCollection 2018. Front Immunol. 2018. PMID: 30283442 Free PMC article. Review.
Cited by
- FABP5 as a possible biomarker in atopic march: FABP5-induced Th17 polarization, both in mouse model and human samples.
Lee J, Kim B, Chu H, Zhang K, Kim H, Kim JH, Kim SH, Pan Y, Noh JY, Sun Z, Lee J, Jeong KY, Park KH, Park JW, Kupper TS, Park CO, Lee KH. Lee J, et al. EBioMedicine. 2020 Aug;58:102879. doi: 10.1016/j.ebiom.2020.102879. Epub 2020 Jul 22. EBioMedicine. 2020. PMID: 32711257 Free PMC article. - Regulation of CD8+ T cells by lipid metabolism in cancer progression.
Tang Y, Chen Z, Zuo Q, Kang Y. Tang Y, et al. Cell Mol Immunol. 2024 Nov;21(11):1215-1230. doi: 10.1038/s41423-024-01224-z. Epub 2024 Oct 14. Cell Mol Immunol. 2024. PMID: 39402302 Free PMC article. Review. - The Biological Functions and Regulatory Mechanisms of Fatty Acid Binding Protein 5 in Various Diseases.
Xu B, Chen L, Zhan Y, Marquez KNS, Zhuo L, Qi S, Zhu J, He Y, Chen X, Zhang H, Shen Y, Chen G, Gu J, Guo Y, Liu S, Xie T. Xu B, et al. Front Cell Dev Biol. 2022 Apr 4;10:857919. doi: 10.3389/fcell.2022.857919. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35445019 Free PMC article. Review. - Gene Expression-Based Identification of Antigen-Responsive CD8+ T Cells on a Single-Cell Level.
Fuchs YF, Sharma V, Eugster A, Kraus G, Morgenstern R, Dahl A, Reinhardt S, Petzold A, Lindner A, Löbel D, Bonifacio E. Fuchs YF, et al. Front Immunol. 2019 Nov 6;10:2568. doi: 10.3389/fimmu.2019.02568. eCollection 2019. Front Immunol. 2019. PMID: 31781096 Free PMC article. - Targeting Resident Memory T Cells for Cancer Immunotherapy.
Blanc C, Hans S, Tran T, Granier C, Saldman A, Anson M, Oudard S, Tartour E. Blanc C, et al. Front Immunol. 2018 Jul 27;9:1722. doi: 10.3389/fimmu.2018.01722. eCollection 2018. Front Immunol. 2018. PMID: 30100906 Free PMC article. Review.
References
- Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI127654/AI/NIAID NIH HHS/United States
- DP5 OD023091/OD/NIH HHS/United States
- R01 AR065807/AR/NIAMS NIH HHS/United States
- R01 CA203721/CA/NCI NIH HHS/United States
- R01 AR063962/AR/NIAMS NIH HHS/United States
- R01 AI041707/AI/NIAID NIH HHS/United States
- P30 AR069625/AR/NIAMS NIH HHS/United States
- R01 AI097128/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous