Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice - PubMed (original) (raw)
doi: 10.1038/ncomms14572.
Jeanne Cheung 1, Armando Navarro 2, Steve Lianoglou 3, Benjamin Haley 4, Klara Totpal 2, Laura Sanders 1, Hartmut Koeppen 5, Patrick Caplazi 5, Jacqueline McBride 6, Henry Chiu 7, Rebecca Hong 2, Jane Grogan 1, Vincent Javinal 2, Robert Yauch 8, Bryan Irving 1, Marcia Belvin 1, Ira Mellman 1, Jeong M Kim 1, Maike Schmidt 1
Affiliations
- PMID: 28220772
- PMCID: PMC5321797
- DOI: 10.1038/ncomms14572
Tumour and host cell PD-L1 is required to mediate suppression of anti-tumour immunity in mice
Janet Lau et al. Nat Commun. 2017.
Abstract
Expression of PD-L1, the ligand for T-cell inhibitory receptor PD-1, is one key immunosuppressive mechanism by which cancer avoids eradication by the immune system. Therapeutic use of blocking antibodies to PD-L1 or its receptor PD-1 has produced unparalleled, durable clinical responses, with highest likelihood of response seen in patients whose tumour or immune cells express PD-L1 before therapy. The significance of PD-L1 expression in each cell type has emerged as a central and controversial unknown in the clinical development of immunotherapeutics. Using genetic deletion in preclinical mouse models, here we show that PD-L1 from disparate cellular sources, including tumour cells, myeloid or other immune cells can similarly modulate the degree of cytotoxic T-cell function and activity in the tumour microenvironment. PD-L1 expression in both the host and tumour compartment contribute to immune suppression in a non-redundant fashion, suggesting that both sources could be predictive of sensitivity to therapeutic agents targeting the PD-L1/PD-1 axis.
Conflict of interest statement
All authors are current or former employees of Genentech Inc., a member of the Roche group.
Figures
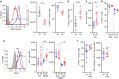
Figure 1. PD-L1 expression in malignant epithelial and immune cells of human tumours.
IHC analysis of human non-small-cell lung cancer (NSCLC) (a) and triple-negative breast cancer (TNBC) (b) samples identified three distinct patterns of PD-L1 expression (brown) in the tumour epithelium, immune cells or both compartments. In mouse tumour models in vivo, PD-L1 RNA and surface protein expression were detectable in MC-38 or CT-26 tumour cells, as well as in myeloid (mye) and lymphoid (lym) cells (c). Treatment of wild-type MC-38 (d) or CT-26 (e) tumours with anti-PD-L1 blocking antibodies resulted in slowed tumour growth and tumour regressions. If not labelled in graph, data shown is from MC38 (open circles) and CT26 (filled circles). Treatment data is representative of multiple independent study repeats with the same antibody. CR, complete regression; TGI, tumour growth inhibition. Scale bar represents 20 μm. Error bars depict s.d. from the mean.
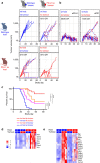
Figure 2. Genetic deletion of PD-L1 results in T-cell activation and tumor regression.
Flow cytometry analysis of PD-L1-deficient hosts showed increased T-cell infiltration and PD-1 expression in MC-38 tumours (a) and reduced tumour growth rate and complete tumour regressions (CR) were observed in 3/10 PD-L1-deficient mice (b). Most significantly upregulated gene sets were indicative of strong T-cell activation in MC38 wt tumor grown in PD-L1-deficient hosts (c). Similarly, wild-type (wt) mice inoculated with PD-L1-deficient MC-38 tumour cells showed increased activated, T-cell infiltration by flow cytometry (e). While PD-L1-deficient MC38 tumour cell inoculated into immune-deficient Rag2 KO mice showed normal tumour outgrowth (d), inoculation into wt mice leads to complete regression (CR) in 4/10 mice (f). Most significantly up-regulated gene sets were distinct and included stromal remodelling mechanisms (g). Significantly modulated chemokine gene expression was distinct between the host versus tumour PD-L1-deficient setting (h). Concordantly with the chemokine changes, increased numbers of granulocytic myeloid derived suppressor cells (MDSC) were detected in PD-L1-deficient tumours by flow cytometry (i). Depleting cytolytic CD8+ populations alleviated PD-L1-deficient tumour regression (j). Characterization of immune subsets by flow cytometry in tumours was performed between day 14–21 post inoculation. Tumour samples for RNA analysis were collected at d9 post inocculation. RNA and flow cytometry data shown is from wt MC-38 tumours in PD-L1-deficient host (crossed circles) or PD-L1-deficient MC-38 tumours in wt host (open circles), with PD-L1 wt status represented in blue and PD-L1 deficiency (ko) represented in red. For tumour growth curves, the symbol colour is representative of tumour cell PD-L1 status, the line colour for host PD-L1 status. Data are representative of a minimum of two independent study repeats. Statistical significance was determined by Student's _t_-test (*P<0.05; **P<0.01.) for individual RNA or protein analytes, by CAMERA FDR P<0.05 for gene set enrichment analysis. Error bars depict s.d. from the mean. Mouse cartoon adapted from ref. .
Figure 3. Reconstituting PD-L1 expression in tumour cells influences tumour outgrowth.
PD-L1-deficient MC38 tumour cells were reconstituted with doxycycline inducible RFP (red circles), or PD-L1 (blue circle) using a lentiviral construct encoding constitutive GFP. PD-L1 reconstitution resulted in restored tumour outgrowth (a), while PD-L1-deficient tumours with doxycycline inducible RFP regressed. End-point analysis at day 24 showed that mixed cell (red/blue circles) inoculation led to tumours slightly smaller compared to tumours generated from PD-L1-enabled tumour cells only (b). While RFP+GFP+ tumour cells were detectable by flow cytometry at time of inoculation, this population was lost at endpoint, and solely PD-L1+GFP+ cells remained from the mixed inoculation (c). PD-L1 on target cells can inhibit antigen specific cytolytic T-cell activity in a dose dependent manner in vitro (d). Following complete regression of PD-L1-deficient CT-26 tumours, mice re-challenged with PD-L1-deficient (red) or wildtype (blue) tumours showed complete tumour regression (e), despite outgrowth of syngeneic EMT6 (black) tumours on the opposing flank (f). Following complete regression of MC38 PD-L1-deficient tumours, mice re-challenged with MC38 tumour cells over-expressing doxycycline induced PD-L1 (light blue) showed near complete tumor regression (g), despite over-expressed PD-L1 enabling slightly faster tumour progression in naive mice (h). Characterization of tumor subsets by flow cytometry in tumours was performed at day 19 post-inoculation. Data is representative of at least two individual study repeats, with 3 to 10 mice/group. Cytolytic in vitro assays were repeated four times with representative data shown. Dox, doxycycline; NA, naive; OVA, ovalbumin. Statistical significance was determined by Student's _t_-test (*P<0.05; **P<0.01; ***P<0.001). Error bars depict s.d. from the mean. Mouse cartoon modified from ref. .
Figure 4. Outgrowing tumours lacking PD-L1 apply various putative escape mechanisms.
Flow cytometry analysis of MC-38 tumours indicated that PD-L1-deficient tumours contained fewer MHC-I positive tumour cells and reduced MHC-I surface expression levels (a), increased frequency of PD-L2 expressing tumour cells (b), and PD-L2 levels on myeloid dendritic cells (DC) (c). Although the percentage of PD-L1 positive myeloid and lymphocyte populations were unchanged (d), PD-L1 levels were notably increased in monocytic myeloid derived suppressor cells (MDSC) isolated from PD-L1-deficient tumours (e). PD-L1 levels on wild-type MC38 tumour cells remained high when implanted in wild-type or PD-L1-deficient hosts (f). Data shown is from wt MC-38 tumours in PD-L1-deficient host (crossed circles) or PD-L1-deficient MC-38 tumours in wt host (open circles), with PD-L1 wt status represented in blue and PD-L1 deficiency (ko) represented in red. Characterization of immune subsets by flow cytometry in in vivo tumours was performed at day 24 post-inoculation. Statistical significance was determined by Student's _t_-test (*P<0.05; **P<0.01, ***P<0.001). Error bars depict s.d. from the mean.
Figure 5. Deletion of PD-L1 from both tumor and host compartments lead to most profound frequency of tumor regressions.
Tumour growth and frequency of regressions of wild-type (blue marker) and PD-L1-deficient (red marker) MC38 tumours in wild-type (blue line) and PD-L1-deficient (red line) host mice (a), with highest frequency of regressions observed when PD-L1 was genetically ablated in the tumour and the host compartment. Statistical significance was analysed in two repeat studies (c: summary of two independent repeat studies with _n_=10 per group per study; Mantel-Cox log-rank test; detailed statistics shown in table 1). Concomitant treatment of PD-L1-deficient tumours with either anti-PD-L1 or anti-PD-1 blocking antibodies increased frequency of tumour regressions (b). Optimal T-cell activation as represented by cytolytic gene expression required deletion of PD-L1 in both tumour and host compartment (d), and alleviated degree of potential resistance mechansims represented by stromal remodelling and EMT gene expression (e). In tumour growth curves, PD-L1 status of the host is the line colour whereas the tumor status is the symbol colour. Gene expression analysis limited to genes meeting Q value<0.05. Error bars depict s.d. from the mean. Mouse cartoon modified from ref. .
Similar articles
- The Tumor Microenvironment Regulates Sensitivity of Murine Lung Tumors to PD-1/PD-L1 Antibody Blockade.
Li HY, McSharry M, Bullock B, Nguyen TT, Kwak J, Poczobutt JM, Sippel TR, Heasley LE, Weiser-Evans MC, Clambey ET, Nemenoff RA. Li HY, et al. Cancer Immunol Res. 2017 Sep;5(9):767-777. doi: 10.1158/2326-6066.CIR-16-0365. Epub 2017 Aug 17. Cancer Immunol Res. 2017. PMID: 28819064 Free PMC article. - Combined Blockade of IL6 and PD-1/PD-L1 Signaling Abrogates Mutual Regulation of Their Immunosuppressive Effects in the Tumor Microenvironment.
Tsukamoto H, Fujieda K, Miyashita A, Fukushima S, Ikeda T, Kubo Y, Senju S, Ihn H, Nishimura Y, Oshiumi H. Tsukamoto H, et al. Cancer Res. 2018 Sep 1;78(17):5011-5022. doi: 10.1158/0008-5472.CAN-18-0118. Epub 2018 Jul 2. Cancer Res. 2018. PMID: 29967259 - PD-1/PD-L1 immune checkpoint: Potential target for cancer therapy.
Dermani FK, Samadi P, Rahmani G, Kohlan AK, Najafi R. Dermani FK, et al. J Cell Physiol. 2019 Feb;234(2):1313-1325. doi: 10.1002/jcp.27172. Epub 2018 Sep 7. J Cell Physiol. 2019. PMID: 30191996 Review. - Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape.
Jiang X, Wang J, Deng X, Xiong F, Ge J, Xiang B, Wu X, Ma J, Zhou M, Li X, Li Y, Li G, Xiong W, Guo C, Zeng Z. Jiang X, et al. Mol Cancer. 2019 Jan 15;18(1):10. doi: 10.1186/s12943-018-0928-4. Mol Cancer. 2019. PMID: 30646912 Free PMC article. Review. - Communication between EMT and PD-L1 signaling: New insights into tumor immune evasion.
Jiang Y, Zhan H. Jiang Y, et al. Cancer Lett. 2020 Jan 1;468:72-81. doi: 10.1016/j.canlet.2019.10.013. Epub 2019 Oct 9. Cancer Lett. 2020. PMID: 31605776 Review.
Cited by
- Immunomodulatory Prodrug Micelles Imitate Mild Heat Effects to Reshape Tumor Microenvironment for Enhanced Cancer Immunotherapy.
Ngo TL, Wang KL, Pan WY, Ruan T, Lin YJ. Ngo TL, et al. ACS Nano. 2024 Feb 20;18(7):5632-5646. doi: 10.1021/acsnano.3c11186. Epub 2024 Feb 12. ACS Nano. 2024. PMID: 38344992 Free PMC article. - Genetic ablation of adipocyte PD-L1 reduces tumor growth but accentuates obesity-associated inflammation.
Wu B, Chiang HC, Sun X, Yuan B, Mitra P, Hu Y, Curiel TJ, Li R. Wu B, et al. J Immunother Cancer. 2020 Aug;8(2):e000964. doi: 10.1136/jitc-2020-000964. J Immunother Cancer. 2020. PMID: 32817394 Free PMC article. - Tumor PD-L1 engages myeloid PD-1 to suppress type I interferon to impair cytotoxic T lymphocyte recruitment.
Klement JD, Redd PS, Lu C, Merting AD, Poschel DB, Yang D, Savage NM, Zhou G, Munn DH, Fallon PG, Liu K. Klement JD, et al. Cancer Cell. 2023 Mar 13;41(3):620-636.e9. doi: 10.1016/j.ccell.2023.02.005. Cancer Cell. 2023. PMID: 36917954 Free PMC article. - Impact of Epigenetic Regulation on Head and Neck Squamous Cell Carcinoma.
Bais MV. Bais MV. J Dent Res. 2019 Mar;98(3):268-276. doi: 10.1177/0022034518816947. Epub 2019 Jan 7. J Dent Res. 2019. PMID: 30615537 Free PMC article. - Adipose PD-L1 Modulates PD-1/PD-L1 Checkpoint Blockade Immunotherapy Efficacy in Breast Cancer.
Wu B, Sun X, Gupta HB, Yuan B, Li J, Ge F, Chiang HC, Zhang X, Zhang C, Zhang D, Yang J, Hu Y, Curiel TJ, Li R. Wu B, et al. Oncoimmunology. 2018 Aug 23;7(11):e1500107. doi: 10.1080/2162402X.2018.1500107. eCollection 2018. Oncoimmunology. 2018. PMID: 30393583 Free PMC article.
References
- Dong H. et al.. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials