Cellular senescence mediates fibrotic pulmonary disease - PubMed (original) (raw)
doi: 10.1038/ncomms14532.
Thomas A White 1, Koji Iijima 3, Andrew J Haak 4, Giovanni Ligresti 4, Elizabeth J Atkinson 5, Ann L Oberg 5, Jodie Birch 6, Hanna Salmonowicz 6, Yi Zhu 1, Daniel L Mazula 1, Robert W Brooks 7, Heike Fuhrmann-Stroissnigg 7, Tamar Pirtskhalava 1, Y S Prakash 4 8, Tamara Tchkonia 1, Paul D Robbins 7, Marie Christine Aubry 9, João F Passos 6, James L Kirkland 1 4 10, Daniel J Tschumperlin 4, Hirohito Kita 3, Nathan K LeBrasseur 1 2 4
Affiliations
- PMID: 28230051
- PMCID: PMC5331226
- DOI: 10.1038/ncomms14532
Cellular senescence mediates fibrotic pulmonary disease
Marissa J Schafer et al. Nat Commun. 2017.
Abstract
Idiopathic pulmonary fibrosis (IPF) is a fatal disease characterized by interstitial remodelling, leading to compromised lung function. Cellular senescence markers are detectable within IPF lung tissue and senescent cell deletion rejuvenates pulmonary health in aged mice. Whether and how senescent cells regulate IPF or if their removal may be an efficacious intervention strategy is unknown. Here we demonstrate elevated abundance of senescence biomarkers in IPF lung, with p16 expression increasing with disease severity. We show that the secretome of senescent fibroblasts, which are selectively killed by a senolytic cocktail, dasatinib plus quercetin (DQ), is fibrogenic. Leveraging the bleomycin-injury IPF model, we demonstrate that early-intervention suicide-gene-mediated senescent cell ablation improves pulmonary function and physical health, although lung fibrosis is visibly unaltered. DQ treatment replicates benefits of transgenic clearance. Thus, our findings establish that fibrotic lung disease is mediated, in part, by senescent cells, which can be targeted to improve health and function.
Conflict of interest statement
Y.Z., T.P., T.T. and J.L.K. declare competing financial interests. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. The remaining authors declare no competing financial interests.
Figures
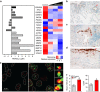
Figure 1. Biomarkers of cellular senescence in human IPF.
(a) Transcriptional changes corresponding to senescence effectors (black), SASP growth factors (dark grey) and SASP matrix remodelling (light grey) genes that were identified in independent RNAseq (control _n_=19, IPF _n_=20) and microarray human lung IPF versus control data sets are shown. IPF samples analysed by microarray were severity classified by FVC as low (≥80%; _n_=17), moderate (50–80%; _n_=60) or severe (<50%; _n_=16) and compared with control (_n_=64) (q<0.05 for both RNAseq and microarray). Human lung tissue sections were IHC stained for p16 in b control and (c,d) IPF lung samples with (c) fibroblastic foci and (d) honeycomb lung depicted. p16-positive fibroblasts (stars) and epithelial cells (arrows) are indicated ( × 200 images). (e) Control (left panel) and IPF (right panel) lung sections were analysed for frequencies of DNA damage foci (γH2A.X, green) and telomere immuno-fluorescence in situ hybridization (red) within alveolar compartments. Arrows indicate γH2A.X foci co-localizing with telomeres (TAF) (scale bar, 5 μm), shown at higher magnification on the right (images are from maximum intensity projection). (f) Mean number of γH2A.X foci (left) and percentage of cells containing at least one TAF (right) were determined through quantification of _Z_-stack images with at least 100 cells per sample ( × 100 images) (mean±s.e.m.; control _n_=10 (grey), IPF _n_=27 (red); _t_-test *_P_≤0.05).
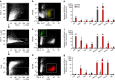
Figure 2. Bleomycin-induced senescence in murine lung cells.
(a–d) Gating strategy for isolation of fibroblasts, epithelial and endothelial cells from the lungs of mice 14 days post-aerosolized instillation of bleomycin (Bleo) or PBS. (a) Total single-cell suspensions (P1) were gated to exclude doublets (P2) and CD45+ cells (P3). (b) Fibroblasts (PDGFRα+, EPCAM−, CD31− and CD45−), (c) epithelial cells (EPCAM+, PDGFRα−, CD31− and CD45−) and (d) endothelial cells (CD31+, PDGFRα−, EPCAM−, CD45−) were sorted from the P3 population. The expression of p16, SASP genes (Mcp1, Pai1, Tnfα, Mmp10, Mmp12) and fibrotic genes (Col1a1 and Tgfβ) were quantified by RT–PCR and are expressed relative to Hprt levels in sorted populations of (e) fibroblasts, (f) epithelial cells and (g) endothelial cells (mean±s.e.m.; PBS _n_=8 (grey), Bleo _n_=6 (red); _t_-test, ***P<0.0005, **P<0.005, *P<0.05, ¥_P_≤0.07 and #_P_≤0.1.).
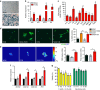
Figure 3. The secretome of senescent fibroblasts is profibrotic.
IMR90 lung fibroblasts were exposed to irradiation (10 Gy). Twenty-one days later, senescence was confirmed by (a) SA-β-gal staining (scale bar, 100 μm) and (b) RT–PCR assessment of p16, p21, MCP1 and IL6 expression relative to TBP levels in 10 Gy exposed (red) and sham-treated (grey) cells (mean±s.e.m.; _n_=3, *_P_≤0.05), as well as (c) immunoanalysis of secreted SASP components within 10 Gy-exposed CM (SASP-CM), relative to CCM (mean±s.e.m.; _n_=4, **P<0.01, *_P_≤0.05, ¥_P_=0.08 and #_P_≤0.1.) (d) IMR90 cells were treated with media collected 21 days post 10 Gy, -sham exposure or control as follows: NCM (black), NCM+2 ng ml−1 TGFβ (orange), CCM (grey) or SASP-CM (red). IMR90 cells were treated with the indicated media for 72 h then immunostained for αSMA (green) and DAPI (blue). Percentage αSMA-positive cells were determined blindly, using a visual threshold (mean±s.e.m.; _n_=2–4 independent experiments; **P<0.01 and *_P_≤0.05). (e) IMR90 cells were plated onto 6.4 kPA matrices for traction force microscopy in the presence of the indicated media for 72 h. Representative traction heat maps, root mean square (RMS) traction and peak traction are depicted (mean±s.e.m.; _n_=2–4 independent experiments with a minimum of 20 independent cells per condition; **P<0.01 and *_P_≤0.05 versus control CM). (f) IMR90 cells were treated with indicated media for 72 h before RNA isolation. RT–PCR expression of ACTA2, COL1A1, COL1A2 and FN1 were measured relative to GAPDH levels (mean±s.e.m.; _n_=2–4 independent experiments, *_P_≤0.05). (g) Human primary lung fibroblasts were exposed to irradiation (10 Gy). Twenty days post irradiation, cells were treated with the indicated concentrations of DQ (yellow) or navitoclax (green). Cell viability 3 days after drug treatment was measured by ATPLite assays and is indicated as a percentage of plating density at day 0 of treatment. (mean±s.e.m.; _n_=4, _t_-test, ***P<0.001, **P<0.01 and *_P_≤0.05).
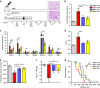
Figure 4. Senescent cell clearance improves pulmonary and physical health in bleomycin injury.
(a) Ink-Attac transgenic mice receiving bleomycin (Bleo) through aerosolized intratracheal instillation were randomized to receive vehicle (Veh), AP or DQ and compared with PBS-exposed mice treated with Veh. Treatment timeline is indicated. Mice were killed 3 weeks post challenge, a time point at which lung fibrosis peaks (haematoxylin and eosin (H&E) panels top: PBS, bottom: Bleo). Lung expression of (b) p16, (c) SASP factors Mcp1, Il6, Tnfα, Mmp3, Mmp12 and profibrotic factors Col1a1 and Tgfβ were quantified by RT–PCR and are expressed relative to Hprt levels. (d) Whole-body plethysmography was used to assess enhanced pause (Penh), an indirect measure of airway resistance. (e) Lung compliance was ascertained by FlexiVent forced oscillation technique at endpoint. (f) Twenty-one-day body weight (BW) was compared with baseline body weight. (g) Exercise capacity was assessed through a treadmill test; distances ran to exhaustion are depicted (mean±s.e.m.; PBS+Veh _n_=13 (grey), Bleo+Veh _n_=8 (red), Bleo+AP _n_=12 (blue), Bleo+DQ _n_=13 (yellow); linear regression model; ***P<0.0005, **P<0.005, *_P_≤0.05, ¥_P_=0.08 and #_P_=0.1).
Figure 5. Retention of p16-positive cells impedes resolution of bleomycin lung injury.
(a) Ink-Attac transgenic mice receiving bleomycin (Bleo) through aerosolized intratracheal instillation were randomized to vehicle (Veh), AP or DQ and were compared with PBS-exposed, Veh-treated mice. Mice were treated as indicated from day 14–28 (grey shading). Body weight (BW) was monitored daily and is depicted as change in grams relative to baseline (*P<0.05). (b) Endpoint (4 weeks post exposure) Penh levels were compared to Penh levels measured at treatment randomization (2 weeks post exposure). Lung p16 expression levels measured by RT–PCR (normalized to Hprt) were compared with endpoint (c) Penh, (d) airway compliance, (e) Mcp1, (f) Pai1, (g) Mmp10 and (h) Col1a1 expression in bleomycin-injured mice (mean±s.e.m.; PBS+Veh _n_=6 (grey), Bleo+Veh _n_=13 (red), Bleo+AP _n_=15 (blue), Bleo+DQ _n_=14 (yellow). Pearson's correlation statistics are indicated).
Comment in
- Licence to kill senescent cells in idiopathic pulmonary fibrosis?
Mailleux AA, Crestani B. Mailleux AA, et al. Eur Respir J. 2017 Aug 3;50(2):1701360. doi: 10.1183/13993003.01360-2017. Print 2017 Aug. Eur Respir J. 2017. PMID: 28775054 No abstract available.
Similar articles
- Senescent lung-resident mesenchymal stem cells drive pulmonary fibrogenesis through FGF-4/FOXM1 axis.
Liu Y, Ji J, Zheng S, Wei A, Li D, Shi B, Han X, Chen X. Liu Y, et al. Stem Cell Res Ther. 2024 Sep 18;15(1):309. doi: 10.1186/s13287-024-03866-2. Stem Cell Res Ther. 2024. PMID: 39289765 Free PMC article. - Quercetin Enhances Ligand-induced Apoptosis in Senescent Idiopathic Pulmonary Fibrosis Fibroblasts and Reduces Lung Fibrosis In Vivo.
Hohmann MS, Habiel DM, Coelho AL, Verri WA Jr, Hogaboam CM. Hohmann MS, et al. Am J Respir Cell Mol Biol. 2019 Jan;60(1):28-40. doi: 10.1165/rcmb.2017-0289OC. Am J Respir Cell Mol Biol. 2019. PMID: 30109946 Free PMC article. - Senolytic drugs target alveolar epithelial cell function and attenuate experimental lung fibrosis ex vivo.
Lehmann M, Korfei M, Mutze K, Klee S, Skronska-Wasek W, Alsafadi HN, Ota C, Costa R, Schiller HB, Lindner M, Wagner DE, Günther A, Königshoff M. Lehmann M, et al. Eur Respir J. 2017 Aug 3;50(2):1602367. doi: 10.1183/13993003.02367-2016. Print 2017 Aug. Eur Respir J. 2017. PMID: 28775044 Free PMC article. - Cell senescence and fibrotic lung diseases.
Liu RM, Liu G. Liu RM, et al. Exp Gerontol. 2020 Apr;132:110836. doi: 10.1016/j.exger.2020.110836. Epub 2020 Jan 17. Exp Gerontol. 2020. PMID: 31958492 Free PMC article. Review. - Fibroblast senescence in the pathology of idiopathic pulmonary fibrosis.
Waters DW, Blokland KEC, Pathinayake PS, Burgess JK, Mutsaers SE, Prele CM, Schuliga M, Grainge CL, Knight DA. Waters DW, et al. Am J Physiol Lung Cell Mol Physiol. 2018 Aug 1;315(2):L162-L172. doi: 10.1152/ajplung.00037.2018. Epub 2018 Apr 26. Am J Physiol Lung Cell Mol Physiol. 2018. PMID: 29696986 Free PMC article. Review.
Cited by
- CD38 in the age of COVID-19: a medical perspective.
Horenstein AL, Faini AC, Malavasi F. Horenstein AL, et al. Physiol Rev. 2021 Oct 1;101(4):1457-1486. doi: 10.1152/physrev.00046.2020. Epub 2021 Mar 31. Physiol Rev. 2021. PMID: 33787351 Free PMC article. Review. - Researching New Drug Combinations with Senolytic Activity Using Senescent Human Lung Fibroblasts MRC-5 Cell Line.
de Godoy MCX, Macedo JA, Gambero A. de Godoy MCX, et al. Pharmaceuticals (Basel). 2024 Jan 4;17(1):70. doi: 10.3390/ph17010070. Pharmaceuticals (Basel). 2024. PMID: 38256903 Free PMC article. - Mesenchymal stem cell-derived extracellular vesicles reduce senescence and extend health span in mouse models of aging.
Dorronsoro A, Santiago FE, Grassi D, Zhang T, Lai RC, McGowan SJ, Angelini L, Lavasani M, Corbo L, Lu A, Brooks RW, Garcia-Contreras M, Stolz DB, Amelio A, Boregowda SV, Fallahi M, Reich A, Ricordi C, Phinney DG, Huard J, Lim SK, Niedernhofer LJ, Robbins PD. Dorronsoro A, et al. Aging Cell. 2021 Apr;20(4):e13337. doi: 10.1111/acel.13337. Epub 2021 Mar 16. Aging Cell. 2021. PMID: 33728821 Free PMC article. - Cellular senescence: Neither irreversible nor reversible.
Reimann M, Lee S, Schmitt CA. Reimann M, et al. J Exp Med. 2024 Apr 1;221(4):e20232136. doi: 10.1084/jem.20232136. Epub 2024 Feb 22. J Exp Med. 2024. PMID: 38385946 Free PMC article. - Senescence and cancer - role and therapeutic opportunities.
Schmitt CA, Wang B, Demaria M. Schmitt CA, et al. Nat Rev Clin Oncol. 2022 Oct;19(10):619-636. doi: 10.1038/s41571-022-00668-4. Epub 2022 Aug 31. Nat Rev Clin Oncol. 2022. PMID: 36045302 Free PMC article. Review.
References
- Ashcroft G. S., Horan M. A. & Ferguson M. W. Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab. Invest. 78, 47–58 (1998). - PubMed
- King T. E. Jr, Pardo A. & Selman M. Idiopathic pulmonary fibrosis. Lancet 378, 1949–1961 (2011). - PubMed
- American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am. J. Respir. Crit. Care Med. 161, 646–664 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG053832/AG/NIA NIH HHS/United States
- R01 HL092961/HL/NHLBI NIH HHS/United States
- BB/H022384/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R37 AG013925/AG/NIA NIH HHS/United States
- UL1 TR000135/TR/NCATS NIH HHS/United States
- R01 AG013925/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases