Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer - PubMed (original) (raw)
. 2017 Mar 6;214(3):579-596.
doi: 10.1084/jem.20162024. Epub 2017 Feb 23.
Daniel Öhlund 1 2 3, Giulia Biffi 1 2, Ela Elyada 1 2, Ana S Almeida 1 4, Mariano Ponz-Sarvise 1 2 5, Vincenzo Corbo 1 2 6 7, Tobiloba E Oni 1 2 8, Stephen A Hearn 1, Eun Jung Lee 1 2, Iok In Christine Chio 1 2, Chang-Il Hwang 1 2, Hervé Tiriac 1 2, Lindsey A Baker 1 2, Dannielle D Engle 1 2, Christine Feig 9, Anne Kultti 9, Mikala Egeblad 1, Douglas T Fearon 1, James M Crawford 10, Hans Clevers 11, Youngkyu Park 1 2, David A Tuveson 1 2
Affiliations
- PMID: 28232471
- PMCID: PMC5339682
- DOI: 10.1084/jem.20162024
Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer
Daniel Öhlund et al. J Exp Med. 2017.
Abstract
Pancreatic stellate cells (PSCs) differentiate into cancer-associated fibroblasts (CAFs) that produce desmoplastic stroma, thereby modulating disease progression and therapeutic response in pancreatic ductal adenocarcinoma (PDA). However, it is unknown whether CAFs uniformly carry out these tasks or if subtypes of CAFs with distinct phenotypes in PDA exist. We identified a CAF subpopulation with elevated expression of α-smooth muscle actin (αSMA) located immediately adjacent to neoplastic cells in mouse and human PDA tissue. We recapitulated this finding in co-cultures of murine PSCs and PDA organoids, and demonstrated that organoid-activated CAFs produced desmoplastic stroma. The co-cultures showed cooperative interactions and revealed another distinct subpopulation of CAFs, located more distantly from neoplastic cells, which lacked elevated αSMA expression and instead secreted IL6 and additional inflammatory mediators. These findings were corroborated in mouse and human PDA tissue, providing direct evidence for CAF heterogeneity in PDA tumor biology with implications for disease etiology and therapeutic development.
© 2017 Öhlund et al.
Figures
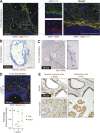
Figure 1.
High expression of αSMA is a distinctive property of periglandular CAFs in mouse and human PDA. (A, left) Representative immunofluorescence (IF) co-staining of FAP (green) and αSMA (red) in a well-differentiated human PDA (n = 4). Counterstain, DAPI (blue). (right) Higher magnification illustrating the distribution and co-localization of FAP and αSMA. Bars, 50 µm. T, tumor glands. (B) Representative image of RNA ISH for Cytokeratin 18 (KRT18, blue) and αSMA (ACTA2, red) transcripts in a well-differentiated human PDA (n = 3). Bar, 50 µm. T, tumor gland. (C, left) Representative image of RNA ISH for Fap (blue) and Acta2 (red) in a KPC mouse tumor (n = 3). (right) Higher magnification. Bars, 25 µm. T, tumor glands. (D, top) Representative image of fluorescent RNA ISH for Fap (green) and Acta2 (red) in a KPC mouse tumor (n = 3), showing transcript distribution across three cell layers of the stroma, starting from the first layer adjacent to the tumor gland (T) and moving outwards. Counterstain, DAPI (blue). Bar, 50 µm. (bottom) Quantification of Fap and Acta2 fluorescence intensity in the three cell layers. Results show mean ± SD of three tumor glands. Data are normalized to layer 1. ***, P < 0.001, unpaired Student’s t test. (E) Representative images of IHC of αSMA and YFP in sequential tissue sections from KPCY mice, with either preinvasive Pancreatic Intraepithelial Neoplasia (PanIN) or invasive cancer (n = 2). Arrows indicate areas of myCAFs. Bar, 50 µm.
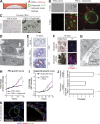
Figure 2.
Co-cultures of mouse PSCs and pancreatic cancer organoids recapitulate properties of PDA desmoplasia. (A) Schematic illustration of the co-culture platform. (B) Representative images of mCherry-labeled PSCs (red) cultured alone or in co-culture with GFP-labeled tumor-derived organoids (green), and imaged by confocal microscopy after 4 or 7 d in bright field and by fluorescent microscopy (n = 3). Arrows point to close interactions between organoids and PSCs. Bars, 100 µm. (C) Bright field images of primary PSCs plated in Matrigel directly after isolation, and either cultured alone or co-cultured with tumor organoids for 5 d (n = 3). Bar, 100 µm. (D) Representative electron microscopy image showing the proximity between organoids and PSCs in co-culture (n = 2). Bar, 5 µm. (E) H&E staining and Masson’s trichrome (MT) staining of fixed and paraffin-embedded organoids cultured alone or in co-culture with PSCs (n = 2). Bar, 50 µm. (F) Representative bright field and IF images of collagen I deposition (red) in organoid cultured alone or in co-culture with PSCs (n = 2). Bar, 200 µm. (G) Representative electron microscopy image of banded collagen fibrils (arrow), with fibril diameters ranging between 24 and 35 nm, in the extracellular space between organoids and PSCs (n = 2). Bar, 1 µm. (H) PSC proliferation curves plotting changes in mCherry intensity over time. Results show mean ± SD of two biological replicates. **, P < 0.01, unpaired Student’s t test. (I) Organoid proliferation curves plotting changes in GFP intensity over time. Results show mean ± SD of three biological replicates. *, P < 0.05, unpaired Student’s t test. (J) Passaging of organoids in different culture conditions in the presence or absence of PSCs. Complete media, DMEM/F12 supplemented with mitogens and growth factors. Reduced media, DMEM + 5% FBS. Red dot indicates the passage number when all organoids were found dead. Green dot indicates surviving organoids when the experiment was terminated. Each dot represents one biological replicate. Bars indicate the average number of passages for each condition. (K) RNA ISH of fixed and sectioned co-cultures for αSMA (Acta2, red) and Krt18 (green) illustrating the spatial distribution of αSMAhigh PSCs in comparison to Krt18+ (green) tumor organoids (n = 2). Counterstain, DAPI. Higher magnification on the right. Bars, 50 µm.
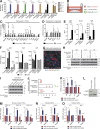
Figure 3.
Secretion of inflammatory cytokines from CAFs activates STAT3 in PDA organoids. (A) Quantification of secretome dot blots of conditioned media from mouse tumor organoid monocultures, PSC monocultures, co-cultures, or Matrigel-only controls (MG). Results show normalized mean ± SD of three biological replicates. (B) Schematic illustration of the trans-well culture platform. (C and D) qPCR analysis of GP130 signaling ligands and receptors in mouse PSCs (C) or tumor organoids (D) cultured in monoculture or trans-well culture. Results show mean ± SD of four biological replicates. n.d., not detected. (E) ELISA of IL-6, IL-11, and LIF from conditioned media of PSC monocultures, organoid monocultures, or co-cultures. Results show mean ± SD of three biological replicates. (F) Quantification of secretome dot blots of conditioned media from human primary CAF monocultures, patient-matched tumor organoid monocultures, or co-cultures (n = 2). Results show mean ± SD of two technical replicates for each condition. (G) Representative IF image of KPC mouse tumor stained for phosphorylated STAT3 (Tyr705; green, pSTAT3) and the epithelial marker Cytokeratin 19 (Krt19, red; n = 2). Counterstain, DAPI (blue). Bar, 75 µm. (H) Western blot analysis of pSTAT3 in organoids treated with either 10 ng/ml recombinant IL-6, 10 ng/ml recombinant IL-11, or 50 ng/ml recombinant LIF, in the presence or absence of neutralizing antibodies or isotype controls (n = 2). Loading control, Actin. Molecular weights in kilodaltons. (I) Western blot analysis of pSTAT3 in organoids treated with co-culture conditioned media in the presence or absence of neutralizing antibodies against IL-6, IL-11, or LIF (n = 3). Loading control, Actin. Molecular weights in kilodaltons. (J) Passaging of organoids in reduced media conditions in monoculture or co-culture with WT (PSC WT) or IL-6 KO PSCs (PSC IL-6 KO). Red dot indicates the passage number when all organoids were found dead. Green dot indicates the passage number of surviving organoids when the experiment was terminated. Each dot represents one biological replicate. Bars indicate the average number of passages for each condition. (K) qPCR analysis of Il6, Il11, Lif, and Acta2 transcript levels in PSCs cultured with control media (Matrigel-only conditioned media) or tumor organoid conditioned media. Results show mean ± SD of five biological replicates for Il6, Lif, and Acta2, and three biological replicates for Il11. (L) Western blot analysis of PSCs cultured with control media or tumor organoid conditioned media (n = 3). Loading control, Hsp90α. Molecular weights in kilodaltons. (M and N) qPCR analysis of Il6 and Acta2 in three primary PSC lines (M) and two KPC mouse CAFs (N) cultured with control media or tumor organoid conditioned media. Results show mean ± SD of two technical replicates for each line. (O) qPCR analysis for IL6 and ACTA2 transcript levels in human primary CAFs cultured with control media or conditioned media from the corresponding patient-matched tumor organoids. Results show mean ± SD of 2 technical replicates. *, P < 0.05; **, P < 0.01; ***, P < 0.001, unpaired Student’s t test.
Figure 4.
Two mutually exclusive subpopulations of CAFs with reversible features coexist in pancreatic cancer. (A and B) Flow cytometric analysis of αSMA and IL-6 in PSCs cultured alone or in either co-culture (A) or trans-well culture (B) with tumor organoids. Red frame indicates the gate defining myCAFs (αSMAhigh IL-6low) and black frame indicates the gate defining iCAFs (αSMAlow IL-6high). Numbers indicate percentage of cells within marked gate. Graphs on the right are showing the fold change induction of myCAFs and iCAFs in co-culture, normalized to PSCs in monoculture. Results show mean ± SD of four (A) or two (B) biological replicates. *, P < 0.05; **, P < 0.01, unpaired Student’s t test. (C) Fluorescent RNA ISH of fixed and sectioned co-cultures for Il6 (red) and Krt18 (green), illustrating the spatial distribution of IL-6+ PSCs (iCAFs) with respect to KRT18+ tumor organoids (n = 2). Higher magnification on the right. Counterstain, DAPI (blue). Arrow indicates example of an iCAF. Bars, 50 µm. (D) qPCR analysis of interleukins (Il6 and Il11) and markers of fibroblast (Pdgfra, Pdgfrb, Acta2, and Fap), epithelial (Krt19) and macrophage (CD11b) lineages in samples of primary cells sorted from KPC mouse tumors. Sorting was performed using three markers: PDGFRα (CD140a) for fibroblasts (n = 3), EpCAM for epithelial cells (n = 3) and CD45 for immune cells (n = 2). Results show mean ± SD of two to three biological replicates. All gene expression changes are statistically significant when compared with the reference population, P < 0.01, unpaired Student’s t test. (E) Representative image of sequential IHC for IL-6 (purple) and PDGFRβ (brown) in a KPC mouse tumor (n = 3). Arrows indicate double positive cells. T, tumor gland. Bars, 50 µm. (F, left) Representative image of sequential IHC for PDGFRβ (gray), IL-6 (brown), and Ki67 (purple) in a KPC mouse tumor (n = 3). Arrows indicate examples of triple positive cells. Bar, 50 µm. (right) Quantification of Ki67 staining in PDGFRβ+/IL-6+ cells (iCAFs), total of 593 cells were counted. (G) Representative image of sequential IHC for IL-6 (brown) and PDGFRβ (purple) in a human PDA (n = 6). Arrowheads indicate double positive cells. T, tumor gland. Bars, 50 µm. (H) Representative image of RNA ISH for Acta2 (blue) and Il6 (red) in KPC mouse tumors (n = 4). Arrows indicate examples of _Acta2_-positive cells in the periglandular area, arrowheads indicate examples of _Il6_-positive cells further away from neoplastic cells. Bar, 50 µm. T, tumor glands. (I) Representative IF image for αSMA (green) and IL-6 (red) in a KPC mouse tumor (n = 3). Counterstain, DAPI (blue). Arrowheads indicate examples of αSMA-positive cells in the periglandular area; * indicates examples of IL-6–positive cells further away from neoplastic cells. Bar, 50 µm. T, tumor glands. (J) qPCR analysis of Il6, Il11, Lif, and Acta2 transcript levels in two PSC lines (PSC4 and PSC5) first grown as monocultures in Matrigel (quiescent PSCs), then transferred to trans-well cultures with tumor organoids (iCAFs), and finally plated as monolayer cultures (myofibroblasts). Results show mean ± SD of two technical replicates for each PSC line. **, P < 0.01; ***, P < 0.001, unpaired Student’s t test.
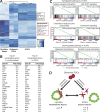
Figure 5.
Inflammatory CAFs and myofibroblasts have distinct transcriptional profiles. (A) RNA sequencing analysis of quiescent PSCs (PSCs embedded alone in Matrigel; n = 2), iCAFs (PSCs grown in trans-well culture with tumor organoids; n = 4) and myofibroblasts (PSCs grown in monolayer; n = 2). The heat map shows differentially expressed genes between the three cell states. Uniquely expressed genes for iCAFs and myofibroblasts are indicated in the boxes. Adjusted P < 0.01. (B) Lists of the 25 most up-regulated genes in iCAFs and myofibroblasts compared with quiescent PSCs. Adjusted P < 0.05. (C) GSEA of most up-regulated and down-regulated pathways in iCAFs compared with quiescent PSCs. (D) Working model illustrating the dynamic relationship between quiescent PSCs, myCAFs and iCAFs.
Similar articles
- IL1-Induced JAK/STAT Signaling Is Antagonized by TGFβ to Shape CAF Heterogeneity in Pancreatic Ductal Adenocarcinoma.
Biffi G, Oni TE, Spielman B, Hao Y, Elyada E, Park Y, Preall J, Tuveson DA. Biffi G, et al. Cancer Discov. 2019 Feb;9(2):282-301. doi: 10.1158/2159-8290.CD-18-0710. Epub 2018 Oct 26. Cancer Discov. 2019. PMID: 30366930 Free PMC article. - Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma.
Neuzillet C, Tijeras-Raballand A, Ragulan C, Cros J, Patil Y, Martinet M, Erkan M, Kleeff J, Wilson J, Apte M, Tosolini M, Wilson AS, Delvecchio FR, Bousquet C, Paradis V, Hammel P, Sadanandam A, Kocher HM. Neuzillet C, et al. J Pathol. 2019 May;248(1):51-65. doi: 10.1002/path.5224. Epub 2019 Feb 22. J Pathol. 2019. PMID: 30575030 Free PMC article. - Recent advances in understanding cancer-associated fibroblasts in pancreatic cancer.
Huang H, Brekken RA. Huang H, et al. Am J Physiol Cell Physiol. 2020 Aug 1;319(2):C233-C243. doi: 10.1152/ajpcell.00079.2020. Epub 2020 May 20. Am J Physiol Cell Physiol. 2020. PMID: 32432930 Free PMC article. Review. - Pharmacologic Normalization of Pancreatic Cancer-Associated Fibroblast Secretome Impairs Prometastatic Cross-Talk With Macrophages.
Samain R, Brunel A, Douché T, Fanjul M, Cassant-Sourdy S, Rochotte J, Cros J, Neuzillet C, Raffenne J, Duluc C, Perraud A, Nigri J, Gigoux V, Bieche I, Ponzo M, Carpentier G, Cascone I, Tomasini R, Schmid HA, Mathonnet M, Nicolle R, Bousquet MP, Martineau Y, Pyronnet S, Jean C, Bousquet C. Samain R, et al. Cell Mol Gastroenterol Hepatol. 2021;11(5):1405-1436. doi: 10.1016/j.jcmgh.2021.01.008. Epub 2021 Jan 20. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33482394 Free PMC article. - Cancer-associated fibroblasts in pancreatic adenocarcinoma.
Pan B, Liao Q, Niu Z, Zhou L, Zhao Y. Pan B, et al. Future Oncol. 2015 Sep;11(18):2603-10. doi: 10.2217/FON.15.176. Epub 2015 Aug 18. Future Oncol. 2015. PMID: 26284509 Review.
Cited by
- DPYD, down-regulated by the potentially chemopreventive agent luteolin, interacts with STAT3 in pancreatic cancer.
Kato H, Naiki-Ito A, Suzuki S, Inaguma S, Komura M, Nakao K, Naiki T, Kachi K, Kato A, Matsuo Y, Takahashi S. Kato H, et al. Carcinogenesis. 2021 Jul 16;42(7):940-950. doi: 10.1093/carcin/bgab017. Carcinogenesis. 2021. PMID: 33640964 Free PMC article. - Microarchitectural mimicking of stroma-induced vasculature compression in pancreatic tumors using a 3D engineered model.
Heinrich MA, Uboldi I, Kuninty PR, Ankone MJK, van Baarlen J, Zhang YS, Jain K, Prakash J. Heinrich MA, et al. Bioact Mater. 2022 Sep 24;22:18-33. doi: 10.1016/j.bioactmat.2022.09.015. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36203956 Free PMC article. - CAFs/tumor cells co-targeting DNA vaccine in combination with low-dose gemcitabine for the treatment of Panc02 murine pancreatic cancer.
Geng F, Dong L, Bao X, Guo Q, Guo J, Zhou Y, Yu B, Wu H, Wu J, Zhang H, Yu X, Kong W. Geng F, et al. Mol Ther Oncolytics. 2022 Jul 31;26:304-313. doi: 10.1016/j.omto.2022.07.008. eCollection 2022 Sep 15. Mol Ther Oncolytics. 2022. PMID: 36090474 Free PMC article. - Activation of STING in pancreatic cancer-associated fibroblasts exerts an antitumor effect by enhancing tumor immunity.
Suzuki Y, Sato T, Sugimori M, Kanemaru Y, Onodera S, Tsuchiya H, Nakamori Y, Tsuyuki S, Ikeda A, Ikeda R, Goda Y, Kaneko H, Irie K, Sue S, Maeda S. Suzuki Y, et al. Sci Rep. 2024 Jul 24;14(1):17071. doi: 10.1038/s41598-024-68061-y. Sci Rep. 2024. PMID: 39048609 Free PMC article. - T-Cell Immunity in Pancreatic Cancer.
Ajina R, Weiner LM. Ajina R, et al. Pancreas. 2020 Sep;49(8):1014-1023. doi: 10.1097/MPA.0000000000001621. Pancreas. 2020. PMID: 32833941 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA188134/CA/NCI NIH HHS/United States
- P20 CA101955/CA/NCI NIH HHS/United States
- R01 CA190092/CA/NCI NIH HHS/United States
- K99 CA204725/CA/NCI NIH HHS/United States
- P50 CA101955/CA/NCI NIH HHS/United States
- U10 CA180944/CA/NCI NIH HHS/United States
- P30 CA045508/CA/NCI NIH HHS/United States
- U01 CA168409/CA/NCI NIH HHS/United States
- F32 CA180717/CA/NCI NIH HHS/United States
- F32 CA192904/CA/NCI NIH HHS/United States
- T32 CA148056/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials