Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia - PubMed (original) (raw)
Clinical Trial
. 2017 Mar 2;376(9):836-847.
doi: 10.1056/NEJMoa1609783.
Anthony Stein 1, Nicola Gökbuget 1, Adele K Fielding 1, Andre C Schuh 1, Josep-Maria Ribera 1, Andrew Wei 1, Hervé Dombret 1, Robin Foà 1, Renato Bassan 1, Önder Arslan 1, Miguel A Sanz 1, Julie Bergeron 1, Fatih Demirkan 1, Ewa Lech-Maranda 1, Alessandro Rambaldi 1, Xavier Thomas 1, Heinz-August Horst 1, Monika Brüggemann 1, Wolfram Klapper 1, Brent L Wood 1, Alex Fleishman 1, Dirk Nagorsen 1, Christopher Holland 1, Zachary Zimmerman 1, Max S Topp 1
Affiliations
- PMID: 28249141
- PMCID: PMC5881572
- DOI: 10.1056/NEJMoa1609783
Clinical Trial
Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia
Hagop Kantarjian et al. N Engl J Med. 2017.
Abstract
Background: Blinatumomab, a bispecific monoclonal antibody construct that enables CD3-positive T cells to recognize and eliminate CD19-positive acute lymphoblastic leukemia (ALL) blasts, was approved for use in patients with relapsed or refractory B-cell precursor ALL on the basis of single-group trials that showed efficacy and manageable toxic effects.
Methods: In this multi-institutional phase 3 trial, we randomly assigned adults with heavily pretreated B-cell precursor ALL, in a 2:1 ratio, to receive either blinatumomab or standard-of-care chemotherapy. The primary end point was overall survival.
Results: Of the 405 patients who were randomly assigned to receive blinatumomab (271 patients) or chemotherapy (134 patients), 376 patients received at least one dose. Overall survival was significantly longer in the blinatumomab group than in the chemotherapy group. The median overall survival was 7.7 months in the blinatumomab group and 4.0 months in the chemotherapy group (hazard ratio for death with blinatumomab vs. chemotherapy, 0.71; 95% confidence interval [CI], 0.55 to 0.93; P=0.01). Remission rates within 12 weeks after treatment initiation were significantly higher in the blinatumomab group than in the chemotherapy group, both with respect to complete remission with full hematologic recovery (34% vs. 16%, P<0.001) and with respect to complete remission with full, partial, or incomplete hematologic recovery (44% vs. 25%, P<0.001). Treatment with blinatumomab resulted in a higher rate of event-free survival than that with chemotherapy (6-month estimates, 31% vs. 12%; hazard ratio for an event of relapse after achieving a complete remission with full, partial, or incomplete hematologic recovery, or death, 0.55; 95% CI, 0.43 to 0.71; P<0.001), as well as a longer median duration of remission (7.3 vs. 4.6 months). A total of 24% of the patients in each treatment group underwent allogeneic stem-cell transplantation. Adverse events of grade 3 or higher were reported in 87% of the patients in the blinatumomab group and in 92% of the patients in the chemotherapy group.
Conclusions: Treatment with blinatumomab resulted in significantly longer overall survival than chemotherapy among adult patients with relapsed or refractory B-cell precursor ALL. (Funded by Amgen; TOWER ClinicalTrials.gov number, NCT02013167 .).
Conflict of interest statement
No other potential conflict of interest relevant to this article was reported.
Figures
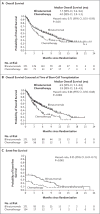
Figure 1. Efficacy End Points
Panel A shows the probability of overall survival in the two groups. Overall survival was calculated as the time from randomization to death from any cause. The median duration of follow-up for overall survival was 11.7 months in the blinatumomab group and 11.8 months in the chemotherapy group. Panel B shows the probability of overall survival in the two groups (also calculated as the time from randomization to death from any cause) when data were censored at the time of allogeneic stem-cell transplantation. The median duration of follow-up for this analysis of overall survival was 7.0 months in the blinatumomab group and 6.0 months in the chemotherapy group. Panel C shows the probability of event-free survival, which was calculated as the time from randomization until relapse after complete remission with full, partial, or incomplete hematologic recovery, or death; patients who did not achieve a complete re-mission with full, partial, or incomplete hematologic recovery were assigned an event-free duration of 1 day. The median duration of follow-up for event-free survival was 7.8 months in the blinatumomab group and 10.2 months in the chemotherapy group. All three analyses were performed in the intention-to-treat population. P values were determined by means of stratified log-rank tests.
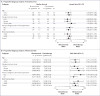
Figure 2. Subgroup Analyses
Panel A shows the results of an analysis of overall survival in prespecified subgroups of the intention-to-treat population that were defined according to baseline characteristics. Overall survival was calculated as the time from randomization to death from any cause. Panel B shows the results of an analysis of remission rates in prespecified subgroups of the intention-to-treat population that were defined according to baseline characteristics. The remission rate was defined as the percentage of patients who had complete hematologic remission with full, partial, or incomplete hematologic recovery by week 12. For both analyses, bone marrow blast data were from the central laboratory, if available; otherwise, data from the local laboratory were used. Central and local baseline results for bone marrow blasts were missing for one patient in the blinatumomab group.
Comment in
- Targeted therapies: New standard for relapsed ALL.
Hutchinson L. Hutchinson L. Nat Rev Clin Oncol. 2017 May;14(5):264. doi: 10.1038/nrclinonc.2017.42. Epub 2017 Mar 21. Nat Rev Clin Oncol. 2017. PMID: 28322244 No abstract available. - Blinatumomab for Acute Lymphoblastic Leukemia.
Mori J, Oshima K, Tanimoto T. Mori J, et al. N Engl J Med. 2017 Jun 8;376(23):e49. doi: 10.1056/NEJMc1704012. N Engl J Med. 2017. PMID: 28594155 No abstract available.
Similar articles
- Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study.
Topp MS, Gökbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA, Neumann S, Foà R, Litzow M, Ribera JM, Rambaldi A, Schiller G, Brüggemann M, Horst HA, Holland C, Jia C, Maniar T, Huber B, Nagorsen D, Forman SJ, Kantarjian HM. Topp MS, et al. Lancet Oncol. 2015 Jan;16(1):57-66. doi: 10.1016/S1470-2045(14)71170-2. Epub 2014 Dec 16. Lancet Oncol. 2015. PMID: 25524800 Clinical Trial. - Effect of Blinatumomab vs Chemotherapy on Event-Free Survival Among Children With High-risk First-Relapse B-Cell Acute Lymphoblastic Leukemia: A Randomized Clinical Trial.
Locatelli F, Zugmaier G, Rizzari C, Morris JD, Gruhn B, Klingebiel T, Parasole R, Linderkamp C, Flotho C, Petit A, Micalizzi C, Mergen N, Mohammad A, Kormany WN, Eckert C, Möricke A, Sartor M, Hrusak O, Peters C, Saha V, Vinti L, von Stackelberg A. Locatelli F, et al. JAMA. 2021 Mar 2;325(9):843-854. doi: 10.1001/jama.2021.0987. JAMA. 2021. PMID: 33651091 Free PMC article. Clinical Trial. - Pediatric posttransplant relapsed/refractory B-precursor acute lymphoblastic leukemia shows durable remission by therapy with the T-cell engaging bispecific antibody blinatumomab.
Schlegel P, Lang P, Zugmaier G, Ebinger M, Kreyenberg H, Witte KE, Feucht J, Pfeiffer M, Teltschik HM, Kyzirakos C, Feuchtinger T, Handgretinger R. Schlegel P, et al. Haematologica. 2014 Jul;99(7):1212-9. doi: 10.3324/haematol.2013.100073. Epub 2014 Apr 11. Haematologica. 2014. PMID: 24727818 Free PMC article. - Blinatumomab: A First-in-Class Bispecific T-Cell Engager for Precursor B-Cell Acute Lymphoblastic Leukemia.
Buie LW, Pecoraro JJ, Horvat TZ, Daley RJ. Buie LW, et al. Ann Pharmacother. 2015 Sep;49(9):1057-67. doi: 10.1177/1060028015588555. Epub 2015 Jun 3. Ann Pharmacother. 2015. PMID: 26041811 Review. - Efficacy and safety of bispecific T-cell engager blinatumomab and the potential to improve leukemia-free survival in B-cell acute lymphoblastic leukemia.
Ribera JM. Ribera JM. Expert Rev Hematol. 2017 Dec;10(12):1057-1067. doi: 10.1080/17474086.2017.1396890. Epub 2017 Nov 1. Expert Rev Hematol. 2017. PMID: 29082835 Review.
Cited by
- Exploring the next generation of antibody-drug conjugates.
Tsuchikama K, Anami Y, Ha SYY, Yamazaki CM. Tsuchikama K, et al. Nat Rev Clin Oncol. 2024 Mar;21(3):203-223. doi: 10.1038/s41571-023-00850-2. Epub 2024 Jan 8. Nat Rev Clin Oncol. 2024. PMID: 38191923 Review. - Tens of images can suffice to train neural networks for malignant leukocyte detection.
Schouten JPE, Matek C, Jacobs LFP, Buck MC, Bošnački D, Marr C. Schouten JPE, et al. Sci Rep. 2021 Apr 12;11(1):7995. doi: 10.1038/s41598-021-86995-5. Sci Rep. 2021. PMID: 33846442 Free PMC article. - Chimeric Antigen Receptor T-Cells in B-Acute Lymphoblastic Leukemia: State of the Art and Future Directions.
Greenbaum U, Mahadeo KM, Kebriaei P, Shpall EJ, Saini NY. Greenbaum U, et al. Front Oncol. 2020 Aug 26;10:1594. doi: 10.3389/fonc.2020.01594. eCollection 2020. Front Oncol. 2020. PMID: 32984022 Free PMC article. Review. - Dual Fc optimization to increase the cytotoxic activity of a CD19-targeting antibody.
Gehlert CL, Rahmati P, Boje AS, Winterberg D, Krohn S, Theocharis T, Cappuzzello E, Lux A, Nimmerjahn F, Ludwig RJ, Lustig M, Rösner T, Valerius T, Schewe DM, Kellner C, Klausz K, Peipp M. Gehlert CL, et al. Front Immunol. 2022 Aug 31;13:957874. doi: 10.3389/fimmu.2022.957874. eCollection 2022. Front Immunol. 2022. PMID: 36119088 Free PMC article. - Breakthrough in Blastic Plasmacytoid Dendritic Cell Neoplasm Cancer Therapy Owing to Precision Targeting of CD123.
Zanotta S, Galati D, De Filippi R, Pinto A. Zanotta S, et al. Int J Mol Sci. 2024 Jan 25;25(3):1454. doi: 10.3390/ijms25031454. Int J Mol Sci. 2024. PMID: 38338733 Free PMC article. Review.
References
- Jabbour E, O’Brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121:2517–28. - PubMed
- Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29:532–43. - PubMed
- Kantarjian H, Thomas D, O’Brien S, et al. Long-term follow-up results of hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone (Hyper-CVAD), a dose-intensive regimen, in adult acute lymphocytic leukemia. Cancer. 2004;101:2788–801. - PubMed
- Gökbuget N, Hoelzer D, Arnold R, et al. Treatment of adult ALL according to protocols of the German Multicenter Study Group for Adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14:1307–25. - PubMed
- Tavernier E, Boiron JM, Huguet F, et al. Outcome of treatment after first relapse in adults with acute lymphoblastic leukemia initially treated by the LALA-94 trial. Leukemia. 2007;21:1907–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources