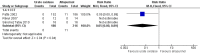Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer - PubMed (original) (raw)
Review
Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer
Daniel Kl Cheuk et al. Cochrane Database Syst Rev. 2017.
Abstract
Background: Tumour lysis syndrome (TLS) is a serious complication of malignancies and can result in renal failure or death. Previous reviews did not find clear evidence of benefit of urate oxidase in children with cancer. This review is the second update of a previously published Cochrane review.
Objectives: To assess the effects and safety of urate oxidase for the prevention and treatment of TLS in children with malignancies.
Search methods: In March 2016 we searched CENTRAL, MEDLINE, Embase, and CINAHL. In addition, we searched the reference lists of all identified relevant papers, trials registers and other databases. We also screened conference proceedings and we contacted experts in the field and the manufacturer of rasburicase, Sanofi-aventis.
Selection criteria: Randomised controlled trials (RCT) and controlled clinical trials (CCT) of urate oxidase for the prevention or treatment of TLS in children under 18 years with any malignancy.
Data collection and analysis: Two review authors independently extracted trial data and assessed individual trial quality. We used risk ratios (RR) for dichotomous data and mean difference (MD) for continuous data.
Main results: We included seven trials, involving 471 participants in the treatment groups and 603 participants in the control groups. No new studies were identified in the update. One RCT and five CCTs compared urate oxidase and allopurinol. Three trials tested Uricozyme, and three trials tested rasburicase for the prevention of TLS.The RCT did not evaluate the primary outcome (incidence of clinical TLS). It showed no clear evidence of a difference in mortality (both all-cause mortality (Fisher's exact test P = 0.23) and mortality due to TLS (no deaths in either group)), renal failure (Fisher's exact test P = 0.46), and adverse effects between the treatment and the control groups (Fisher's exact test P = 1.0). The frequency of normalisation of uric acid at four hours (10 out of 10 participants in the treatment group versus zero out of nine participants in the control group, Fisher's exact test P < 0.001) and area under the curve of uric acid at four days (MD -201.00 mg/dLhr, 95% CI -258.05 mg/dLhr to -143.95 mg/dLhr; P < 0.00001) were significantly better in the treatment group.One CCT evaluated the primary outcome; no clear evidence of a difference was identified between the treatment and the control groups (RR 0.77, 95% CI 0.44 to 1.33; P = 0.34). Pooled results of three CCTs showed significantly lower mortality due to TLS in the treatment group (RR 0.05, 95% CI 0.00 to 0.89; P = 0.04); no clear evidence of a difference in all-cause mortality was identified between the groups (RR 0.19, 95% CI 0.01 to 3.42; P = 0.26). Pooled results from five CCTs showed significantly lower incidence of renal failure in the treatment group (RR 0.26, 95% CI 0.08 to 0.89; P = 0.03). Results of CCTs also showed significantly lower uric acid in the treatment group at two days (three CCTs: MD -3.80 mg/dL, 95% CI -7.37 mg/dL to -0.24 mg/dL; P = 0.04), three days (two CCTs: MD -3.13 mg/dL, 95% CI -6.12 mg/dL to -0.14 mg/dL; P = 0.04), four days (two CCTs: MD -4.60 mg/dL, 95% CI -6.39 mg/dL to -2.81 mg/dL; P < 0.00001), and seven days (one CCT: MD -1.74 mg/dL, 95% CI -3.01 mg/dL to -0.47 mg/dL; P = 0.007) after therapy, but not one day (three CCTs: MD -3.00 mg/dL, 95% CI -7.61 mg/dL to 1.60 mg/dL; P = 0.2), five days (one CCT: MD -1.02 mg/dL, 95% CI -2.24 mg/dL to 0.20 mg/dL; P = 0.1), and 12 days (one CCT: MD -0.80 mg/dL, 95% CI -2.51 mg/dL to 0.91 mg/dL; P = 0.36) after therapy. Pooled results from three CCTs showed higher frequency of adverse effects in participants who received urate oxidase (RR 9.10, 95% CI 1.29 to 64.00; P = 0.03).Another included RCT, with 30 participants, compared different doses of rasburicase (0.2 mg/kg versus 0.15 mg/kg). The primary outcome was not evaluated. No clear evidence of a difference in mortality (all-cause mortality (Fisher's exact test P = 1.0) and mortality due to TLS (no deaths in both groups)) and renal failure (no renal failure in both groups) was identified. It demonstrated no clear evidence of a difference in uric acid normalisation (RR 1.07, 95% CI 0.89 to 1.28; P = 0.49) and uric acid level at four hours (MD 8.10%, 95% CI -0.99% to 17.19%; P = 0.08). Common adverse events of urate oxidase included hypersensitivity, haemolysis, and anaemia, but no clear evidence of a difference between treatment groups was identified (RR 0.54, 95% CI 0.12 to 2.48; P = 0.42).The quality of evidence ranks from very low to low because of imprecise results, and all included trials were highly susceptible to biases.
Authors' conclusions: Although urate oxidase might be effective in reducing serum uric acid, it is unclear whether it reduces clinical TLS, renal failure, or mortality. Adverse effects might be more common for urate oxidase compared with allopurinol. Clinicians should weigh the potential benefits of reducing uric acid and uncertain benefits of preventing mortality or renal failure from TLS against the potential risk of adverse effects.
Conflict of interest statement
Daniel KL Cheuk: nothing to declare Alan KS Chiang: nothing to declare Godfrey CF Chan: nothing to declare Shau Yin Ha: nothing to declare
Figures
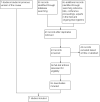
1
Study flow diagram of review update
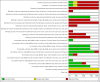
2
'Risk of bias' graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies
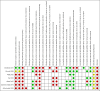
3
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
4
Forest plot of comparison: 1 Urate oxidase compared with allopurinol, outcome: 1.1 Incidence of clinical tumour lysis syndrome
5
Forest plot of comparison: 1 Urate oxidase compared with allopurinol, outcome: 1.4 Mortality due to tumour lysis syndrome
6
Forest plot of comparison: 1 Urate oxidase compared with allopurinol, outcome: 1.5 Renal failure requiring renal replacement therapy
1.1. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 1 Incidence of clinical tumour lysis syndrome.
1.2. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 2 Incidence of clinical tumour lysis syndrome in B‐ALL subgroup.
1.3. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 3 All‐cause mortality.
1.4. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 4 Mortality due to tumour lysis syndrome.
1.5. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 5 Renal failure requiring renal replacement therapy.
1.6. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 6 Renal failure requiring renal replacement therapy in B‐ALL subgroup.
1.7. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 7 AUC of serum uric acid level at 4 days.
1.8. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 8 AUC of serum uric acid level at 4 days in leukaemia participants.
1.9. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 9 AUC of serum uric acid level at 4 days in lymphoma participants.
1.10. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 10 AUC of serum uric acid level at 4 days in hyperuricemic participants.
1.11. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 11 AUC of serum uric acid level at 4 days in normouricemic participants.
1.12. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 12 Serum uric acid level at 2 days.
1.13. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 13 Serum uric acid level at 3 days.
1.14. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 14 Serum uric acid level at 4 days.
1.15. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 15 Serum uric acid level at 7 days.
1.16. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 16 Serum uric acid level at 1 day.
1.17. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 17 Serum uric acid level at 5 days.
1.18. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 18 Serum uric acid level at 12 days.
1.19. Analysis
Comparison 1 Urate oxidase compared with allopurinol, Outcome 19 Frequency of adverse events.
2.1. Analysis
Comparison 2 High‐dose urate oxidase compared with low‐dose urate oxidase, Outcome 1 Normalisation of serum uric acid.
2.2. Analysis
Comparison 2 High‐dose urate oxidase compared with low‐dose urate oxidase, Outcome 2 Percentage reduction in serum uric acid level at 4 hours.
2.3. Analysis
Comparison 2 High‐dose urate oxidase compared with low‐dose urate oxidase, Outcome 3 Frequency of adverse events.
Update of
- Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer.
Cheuk DK, Chiang AK, Chan GC, Ha SY. Cheuk DK, et al. Cochrane Database Syst Rev. 2014 Aug 14;(8):CD006945. doi: 10.1002/14651858.CD006945.pub3. Cochrane Database Syst Rev. 2014. PMID: 25121561 Updated. Review.
Similar articles
- Urate oxidase for the prevention and treatment of tumour lysis syndrome in children with cancer.
Cheuk DK, Chiang AK, Chan GC, Ha SY. Cheuk DK, et al. Cochrane Database Syst Rev. 2014 Aug 14;(8):CD006945. doi: 10.1002/14651858.CD006945.pub3. Cochrane Database Syst Rev. 2014. PMID: 25121561 Updated. Review. - Urate oxidase for the prevention and treatment of tumor lysis syndrome in children with cancer.
Cheuk DK, Chiang AK, Chan GC, Ha SY. Cheuk DK, et al. Cochrane Database Syst Rev. 2010 Jun 16;(6):CD006945. doi: 10.1002/14651858.CD006945.pub2. Cochrane Database Syst Rev. 2010. PMID: 20556770 Updated. Review. - Uricosuric medications for chronic gout.
Kydd AS, Seth R, Buchbinder R, Edwards CJ, Bombardier C. Kydd AS, et al. Cochrane Database Syst Rev. 2014 Nov 14;2014(11):CD010457. doi: 10.1002/14651858.CD010457.pub2. Cochrane Database Syst Rev. 2014. PMID: 25392987 Free PMC article. - Allopurinol for chronic gout.
Seth R, Kydd AS, Buchbinder R, Bombardier C, Edwards CJ. Seth R, et al. Cochrane Database Syst Rev. 2014 Oct 14;2014(10):CD006077. doi: 10.1002/14651858.CD006077.pub3. Cochrane Database Syst Rev. 2014. PMID: 25314636 Free PMC article. Review. - Rasburicase in tumor lysis syndrome of the adult: a systematic review and meta-analysis.
Lopez-Olivo MA, Pratt G, Palla SL, Salahudeen A. Lopez-Olivo MA, et al. Am J Kidney Dis. 2013 Sep;62(3):481-92. doi: 10.1053/j.ajkd.2013.02.378. Epub 2013 May 14. Am J Kidney Dis. 2013. PMID: 23684124 Review.
Cited by
- Evaluation of the safety and efficacy of low-dose rasburicase in critically ill children with haematological malignancies.
Pei Y, Li Y, Liang Y, Xu L, Huang X, Li Y, Tang W, Jiang X. Pei Y, et al. Int J Clin Pharm. 2020 Dec;42(6):1440-1446. doi: 10.1007/s11096-020-01144-8. Epub 2020 Sep 24. Int J Clin Pharm. 2020. PMID: 32974856 Free PMC article. - Tumor lysis syndrome in childhood malignancies.
Cheung WL, Hon KL, Fung CM, Leung AK. Cheung WL, et al. Drugs Context. 2020 Feb 25;9:2019-8-2. doi: 10.7573/dic.2019-8-2. eCollection 2020. Drugs Context. 2020. PMID: 32158483 Free PMC article. Review. - A Case of Type 2 Hypersensitivity to Rasburicase Diagnosed with a Natural Killer Cell Activation Assay.
Viel S, Pescarmona R, Belot A, Nosbaum A, Lombard C, Walzer T, Bérard F. Viel S, et al. Front Immunol. 2018 Jan 29;9:110. doi: 10.3389/fimmu.2018.00110. eCollection 2018. Front Immunol. 2018. PMID: 29434608 Free PMC article. - Effectiveness of fractionated rituximab in preventing tumor lysis syndrome in aggressive B-cell lymphoma: Insights from real-life clinical practice.
Mohamad J, Bouroumeau A, McKee TA, Mach N, Samii K, Chamuleau M, Stenner F, Tamburini J, Lang N. Mohamad J, et al. Cancer Rep (Hoboken). 2024 Oct;7(10):e1983. doi: 10.1002/cnr2.1983. Cancer Rep (Hoboken). 2024. PMID: 39410860 Free PMC article. - Allopurinol use leading to xanthine nephrolithiasis in pediatric tumor lysis syndrome: a case series.
Goswami S, Hanson AE, Rajadhyaksha E, Dangle PP, Schwaderer AL. Goswami S, et al. Pediatr Nephrol. 2024 Nov;39(11):3217-3219. doi: 10.1007/s00467-024-06413-6. Epub 2024 Jun 6. Pediatr Nephrol. 2024. PMID: 38842722
References
References to studies included in this review
Goldman 2001 {published data only}
- Goldman SC, Holcenberg JS, Finklestein JZ, Hutchinson R, Kreissman S, Johnson FL, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood 2001;97(10):2998‐3003. - PubMed
Kikuchi 2009 {published data only}
- Kikuchi A, Kigasawa H, Tsurusawa M, Kawa K, Kikuta A, Tsuchida M, et al. A study of rasburicase for the management of hyperuricemia in pediatric patients with newly diagnosed hematologic malignancies at high risk for tumor lysis syndrome. International Journal of Hematology 2009;90(4):492‐500. - PubMed
Patte 2002 {published data only}
- Patte C, Sakiroglu C, Ansoborlo S, Baruchel A, Plouvier E, Pacquement H, et al. Urate‐oxidase in the prevention and treatment of metabolic complications in patients with B‐cell lymphoma and leukemia, treated in the Société Française d'Oncologie Pédiatrique LMB89 protocol. Annals of Oncology 2002;13(5):789‐95. - PubMed
Pui 1997 {published data only}
- Pui CH, Relling MV, Lascombes F, Harrison PL, Struxiano A, Mondesir JM, et al. Urate oxidase in prevention and treatment of hyperuricemia associated with lymphoid malignancies. Leukemia 1997;11(11):1813‐6. - PubMed
Rényi 2007 {published data only}
- Rényi I, Bárdi E, Udvardi E, Kovács G, Bartyik K, Kajtár P, et al. Prevention and treatment of hyperuricemia with rasburicase in children with leukemia and non‐Hodgkin's lymphoma. Pathology Oncology Research 2007;13(1):57‐62. - PubMed
Sánchez Tatay 2010 {published data only}
- Sánchez Tatay V, López Castilla JD, Carmona Ponce JM, Pérez Hurtado JM, Quiroga Cantero E, Loscertales Abril M. Rasburicase versus allopurinol in the treatment of hyperuricaemia in tumour lysis syndrome [Rasburicasa versus alopurinol como tratamientode la hiperuricemia en el sı´ndrome de lisis tumoral]. Anales de Pediatría 2010;72(2):103‐10. - PubMed
Wössmann 2003 {published data only}
- Wössmann W, Schrappe M, Meyer U, Zimmermann M, Reiter A. Incidence of tumor lysis syndrome in children with advanced stage Burkitt's lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Annals of Hematology 2003;82(3):160‐5. - PubMed
References to studies excluded from this review
Cortes 2010 {published data only}
- Cortes J, Moore JO, Maziarz RT, Wetzler M, Craig M, Matous J, et al. Control of plasma uric acid in adults at risk for tumor lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone‐results of a multicenter phase III study. Journal of Clinical Oncology 2010;28(27):4207‐13. - PMC - PubMed
Ishizawa 2009 {published data only}
Vadhan‐Raj 2012 {published data only}
References to ongoing studies
NCT00199043 {published data only}
- NCT00199043. Randomised phase III trial of effectivity and safety of rasburicase compared with allopurinol for treatment of hyperuricemia in patients with acute lymphoblastic leukemia or high‐grade NHL with high risk of tumor lysis syndrome (> 15 yrs). clinicaltrials.gov/ct2/show/NCT00199043?term=NCT00199043&rank=1 (accessed 26 February 2013).
NCT01200485 {published data only}
- NCT01200485. A randomized phase 2 study to evaluate the efficacy of rasburicase in patients at risk for TLS during two cycles of chemotherapy. clinicaltrials.gov/ct2/show/NCT01200485?term=NCT01200485&rank=1 (accessed 26 February 2013).
Additional references
Annemans 2003a
- Annemans L, Moeremans K, Lamotte M, Garcia Conde J, Berg H, Myint H, et al. Incidence, medical resource utilisation and costs of hyperuricemia and tumour lysis syndrome in patients with acute leukaemia and non‐Hodgkin's lymphoma in four European countries. Leukemia & Lymphoma 2003;44(1):77‐83. - PubMed
Annemans 2003b
- Annemans L, Moeremans K, Lamotte M, Garcia Conde J, Berg H, Myint H, et al. Pan‐European multicentre economic evaluation of recombinant urate oxidase (rasburicase) in prevention and treatment of hyperuricaemia and tumour lysis syndrome in haematological cancer patients. Supportive Care in Cancer 2003;11(4):249‐57. - PubMed
Bose 2011
- Bose P, Qubaiah O. A review of tumour lysis syndrome with targeted therapies and the role of rasburicase. Journal of Clinical Pharmacy and Therapeutics 2011;36(3):299‐326. - PubMed
Bosly 2003
- Bosly A, Sonet A, Pinkerton CR, McCowage G, Bron D, Sanz MA, et al. Rasburicase (recombinant urate oxidase) for the management of hyperuricemia in patients with cancer: report of an international compassionate use study. Cancer 2003;98(5):1048‐54. - PubMed
Cairo 2004
- Cairo MS, Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. British Journal of Haematology 2004;127(1):3‐11. - PubMed
Cairo 2010
- Cairo MS, Coiffier B, Reiter A, Younes A, TLS Expert Panel. Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. British Journal of Haematology 2010;149(4):578‐86. - PubMed
Coiffier 2003
- Coiffier B, Mounier N, Bologna S, Fermé C, Tilly H, Sonet A, et al. Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non‐Hodgkin's lymphoma: results of the GRAAL1 (Groupe d'Etude des Lymphomes de l'Adulte Trial on Rasburicase Activity in Adult Lymphoma) study. Journal of Clinical Oncology 2003;21(23):4402‐6. - PubMed
Coiffier 2008
- Coiffier B, Altman A, Pui CH, Younes A, Cairo MS. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence‐based review. Journal of Clinical Oncology 2008;26(16):2767‐78. - PubMed
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Egger 1997
GRADEpro 2015 [Computer program]
- McMaster University, Evidence Prime, Inc.. GRADEpro Guideline Development Tool. McMaster University, Evidence Prime, Inc., 2015.
Greene 1969
- Greene ML, Fujimoto WY, Seegmiller JE. Urinary xanthine stones ‐ a rare complication of allopurinol therapy. The New England Journal of Medicine 1969;280(8):426‐7. - PubMed
Gutierrez‐Macias 2005
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. - PubMed
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hummel 2003
- Hummel M, Buchheidt D, Reiter S, Bergmann J, Hofheinz R, Hehlmann R. Successful treatment of hyperuricemia with low doses of recombinant urate oxidase in four patients with hematologic malignancy and tumor lysis syndrome. Leukemia 2003;17(12):2542‐4. - PubMed
Hutcherson 2006
- Hutcherson DA, Gammon DC, Bhatt MS, Faneuf M. Reduced‐dose rasburicase in the treatment of adults with hyperuricemia associated with malignancy. Pharmacotherapy 2006;26(2):242‐7. - PubMed
Jeha 2005
- Jeha S, Kantarjian H, Irwin D, Shen V, Shenoy S, Blaney S, et al. Efficacy and safety of rasburicase, a recombinant urate oxidase (Elitek), in the management of malignancy‐associated hyperuricemia in pediatric and adult patients: final results of a multicenter compassionate use trial. Leukemia 2005;19(1):34‐8. - PubMed
Kremer 2016
- Kremer LCM, Jellema P, Leclercq E, Noorman JK, Dalen EC. Cochrane Childhood Cancer Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2016:Issue 7. Art. No.: CHILDCA.
Lascombes 1998
- Lascombes F, Sommelet D, Gebhard F, Leverger G, Schaison G, Pinkerton R, et al. High efficacy of recombinant urate oxidase in prevention of renal failure related to tumor lysis syndrome. Blood 1998;92(Suppl 1):237b.
Lee 2003
- Lee AC, Li CH, So KT, Chan R. Treatment of impending tumor lysis with single‐dose rasburicase. The Annals of Pharmacotherapy 2003;37(11):1614‐7. - PubMed
Liu 2005
- Liu CY, Sims‐McCallum RP, Schiffer CA. A single dose of rasburicase is sufficient for the treatment of hyperuricemia in patients receiving chemotherapy. Leukemia Research 2005;29(4):463‐5. - PubMed
McDonnell 2006
- McDonnell AM, Lenz KL, Frei‐Lahr DA, Hayslip J, Hall PD. Single‐dose rasburicase 6 mg in the management of tumor lysis syndrome in adults. Pharmacotherapy 2006;26(6):806‐12. - PubMed
Navolanic 2003
- Navolanic PM, Pui CH, Larson RA, Bishop MR, Pearce TE, Cairo MS, et al. Elitek‐rasburicase: an effective means to prevent and treat hyperuricemia associated with tumor lysis syndrome, a Meeting Report, Dallas, Texas, January 2002. Leukemia 2003;17(3):499‐514. - PubMed
Pui 2001a
- Pui CH, Mahmoud HH, Wiley JM, Woods GM, Leverger G, Camitta B, et al. Recombinant urate oxidase for the prophylaxis or treatment of hyperuricemia in patients with leukemia or lymphoma. Journal of Clinical Oncology 2001;19(3):697‐704. - PubMed
Pui 2001b
- Pui CH, Jeha S, Irwin D, Camitta B. Recombinant urate oxidase (rasburicase) in the prevention and treatment of malignancy‐associated hyperuricemia in pediatric and adult patients: results of a compassionate‐use trial. Leukemia 2001;15(10):1505‐9. - PubMed
Rampello 2006
- Rampello E, Fricia T, Malaguarnera M. The management of tumor lysis syndrome. Nature Clinical Practice Oncology 2006;3(8):438‐47. - PubMed
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sanofi 2011
- Sanofi‐Synthelabo. Elitek (rasburicase) product monograph. products.sanofi‐aventis.us/elitek/elitek.html (accessed 23 July 2012).
Schünemann 2011a
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Schünemann 2011b
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al (editors). Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Shin 2006
- Shin HY, Kang HJ, Park ES, Choi HS, Ahn HS, Kim SY, et al. Recombinant urate oxidase (rasburicase) for the treatment of hyperuricemia in pediatric patients with hematologic malignancies: results of a compassionate prospective multicenter study in Korea. Pediatric Blood and Cancer 2006;46(4):439‐45. - PubMed
SPSS 2010 [Computer program]
- IBM Corporation. IBM SPSS Statistics for Windows, Version 19.0. Armonk, NY: IBM Corporation, 2010.
Tosi 2008
- Tosi P, Barosi G, Lazzaro C, Liso V, Marchetti M, Morra E, et al. Consensus conference on the management of tumor lysis syndrome. Haematologica 2008;93(12):1877‐85. - PubMed
Trifilio 2006
- Trifilio S, Gordon L, Singhal S, Tallman M, Evens A, Rashid K, et al. Reduced‐dose rasburicase (recombinant xanthine oxidase) in adult cancer patients with hyperuricemia. Bone Marrow Transplantation 2006;37(11):997‐1001. - PubMed
Wang 2006
- Wang LY, Shih LY, Chang H, Jou ST, Lin KH, Yeh TC, et al. Recombinant urate oxidase (rasburicase) for the prevention and treatment of tumor lysis syndrome in patients with hematologic malignancies. Acta Haematologica 2006;115(1‐2):35‐8. - PubMed
Yim 2003
- Yim BT, Sims‐McCallum RP, Chong PH. Rasburicase for the treatment and prevention of hyperuricemia. The Annals of Pharmacotherapy 2003;37(7‐8):1047‐54. - PubMed
References to other published versions of this review
Cheuk 2008
- Cheuk DKL, Chiang AKS, Chan GCF, Ha SY. Urate oxidase for prevention and treatment of tumor lysis syndrome in children with cancer (Protocol). Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006945] - DOI
Cheuk 2010
Cheuk 2014
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical